Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
(2S,5R)-2-Methylaminomethyl-1-methyl-5-phenylpyrrolidine, a chiral diamine ligand for copper(II)-catalysed Henry reactions with superb enantiocontrol†
Dagmar
Scharnagel‡
,
Felix
Prause‡
,
Johannes
Kaldun‡
,
Robert G.
Haase
and
Matthias
Breuning
*
Organic Chemistry Laboratory, University of Bayreuth, Universitätsstraße 30, 95447 Bayreuth, Germany. E-mail: matthias.breuning@uni-bayreuth.de
First published on 6th May 2014
Abstract
A cis-2-aminomethyl-5-phenylpyrrolidine, which is easily available from methyl Boc-L-pyroglutamate, was found to be a highly efficient chiral ligand for Cu(II)-catalysed Henry reactions. Excellent yields (>90%) and superb levels of enantiocontrol (98.5–99.6% ee) were reached with aromatic, heteroaromatic, vinylic, and aliphatic aldehydes (36 examples).
The Henry (or nitroaldol) reaction is a powerful tool for C–C bond formation, because it permits rapid access to valuable synthetic intermediates such as 1,2-amino alcohols and α-hydroxy acids.1 Tremendous advances have been made over the last two decades in the development of enantioselective versions of this reaction.2 Among the many highly efficient systems based on heterobimetal,3 transition metal,4–6 organo7 and enzyme8 catalysis, chirally modified copper complexes have received particular attention due to the wide structural variability of the ligands (diamines, amino alcohols, amino imines, amino pyridines, imino pyridines, Schiff bases, box-type ligands, salen-type ligands, and others),5,6 the ease of preparation and the, in part, high levels of stereocontrol reached. Several of these catalysts permit 99% ee in the addition of nitromethane to some of the aldehydes tested,5 but none is capable of providing 99% ee for the majority of substrates. Herein we present the first copper catalyst that fulfils this demand, giving, for the addition of nitromethane to a broad range of aldehydes, the corresponding β-nitro alcohols in high yield and excellent 99% ee.
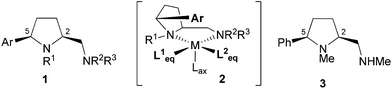
In the course of our studies on bicyclic diamines9 we became interested in 2-aminomethylpyrrolidines of general type 1 (Fig. 1), which carry an additional cis-aryl group in 5-position, as compared to proline derived diamines. Chelation of a metal with 1 will lead to a rigid bicyclic system, in which the aryl substituent is forced into an endo-position directly on top of the active metal site. As illustrated by complex 2, such a shielding might be of particular importance in asymmetric transition metal catalysts preferring Jahn–Teller distorted octahedral geometries, because it selectively blocks one apical position and thereby reduces the number of possible transition states. The equatorial coordination sites L1eq and L2eq are still differentiated by the intrinsic steric and electronic properties of the C1-symmetric diamine 1, which might offer another advantage over C2-symmetric ligands.
Copper(II)-catalysed Henry reactions, which are supposed to proceed via such a pentacoordinate intermediate,10 might provide an ideal test system to probe the potential of the diamines 1.11 After investigating some derivatives, we quickly identified the simple compound 3 as the ligand of choice for these reactions.12
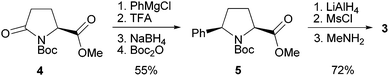
Diamine 3 is easily accessible from commercially available methyl Boc-L-pyroglutamate (4, Scheme 1). Treatment of 4 with phenylmagnesium chloride and re-cyclisation of the resulting, ring-opened ketone delivered the diastereomerically pure pyrrolidine 5 after crystallization.13 Exhaustive reduction followed by OH/NHMe exchange afforded the target molecule 3 in overall seven simple steps and 40% yield.
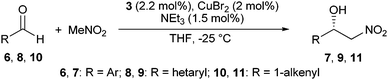
The enantioselective Henry reactions between the aromatic aldehydes 6a–u and nitromethane (11 equivalents) were performed on a 1 mmol scale in THF at −25 °C (Table 1, entries 1–21). The copper(II) complex [3·CuBr2], prepared prior to use by stirring CuBr2 with a slight excess of pyrrolidine 3 in THF, was used as the chiral catalyst and NEt3 (1.5 mol%) as the base. Under these conditions12 and in the presence of just 2 mol% [3·CuBr2], the Henry products 7a–u were formed within 18 to 67 h in excellent 92–99% yield. Outstanding 99% ee, in several cases even more than 99.5% ee, were obtained with electronically more or less neutral (6a–g), electron-deficient (6h–o) and electron-rich (6p–u) aromatic aldehydes, carrying substituents in ortho-, meta-, or para-position.
| Entry | Compounds | R | Time (h) | Yieldb (%) | eec (%) (config.) |
|---|---|---|---|---|---|
| a Performed on a 1 mmol scale in THF (600 μL) and MeNO2 (600 μL ≈ 11 eq.). b Isolated yield. c Determined by HPLC analysis on a chiral phase; absolute configurations were established by comparison with literature data. d Absolute configuration was assigned based on a re-face attack on the aldehyde. | |||||
| 1 | 6a, 7a | Ph | 24 | 92 | 99.3 (S) |
| 2 | 6b, 7b | 2-Me-Ph | 18 | 99 | 99.2 (S) |
| 3 | 6c, 7c | 3-Me-Ph | 20 | 99 | 99.5 (S) |
| 4 | 6d, 7d | 4-Me-Ph | 22 | 93 | 99.4 (S) |
| 5 | 6e, 7e | 4-Ph-Ph | 38 | 99 | 99.6 (S) |
| 6 | 6f, 7f | 1-Naphthyl | 65 | 99 | 99.4 (S) |
| 7 | 6g, 7g | 2-Naphthyl | 42 | 99 | 99.0 (S) |
| 8 | 6h, 7h | 2-O2N-Ph | 20 | 97 | 99.0 (S) |
| 9 | 6i, 7i | 3-O2N-Ph | 22 | 95 | 99.4 (S) |
| 10 | 6j, 7j | 4-O2N-Ph | 21 | 94 | 99.4 (S) |
| 11 | 6k, 7k | 2-Cl-Ph | 18 | 99 | 99.6 (S) |
| 12 | 6l, 7l | 3-Cl-Ph | 19 | 96 | 99.5 (S) |
| 13 | 6m, 7m | 4-Cl-Ph | 42 | 95 | 99.5 (S) |
| 14 | 6n, 7n | 4-F-Ph | 20 | 99 | 99.6 (S) |
| 15 | 6o, 7o | 4-NC-Ph | 21 | 94 | 99.6 (S) |
| 16 | 6p, 7p | 2-MeO-Ph | 42 | 97 | 99.5 (S) |
| 17 | 6q, 7q | 3-MeO-Ph | 48 | 99 | 99.3 (S) |
| 18 | 6r, 7r | 4-MeO-Ph | 67 | 99 | 99.2 (S) |
| 19 | 6s, 7s | 2,4-(MeO)2-Ph | 48 | 98 | 99.3 (S) |
| 20 | 6t, 7t | 2,5-(MeO)2-Ph | 39 | 99 | 99.6 (S) |
| 21 | 6u, 7u | 3,4-(MeO)2-Ph | 40 | 93 | 99.1 (S) |
| 22 | 8a, 9a | 2-Furyl | 40 | 91 | 99.6 (R) |
| 23 | 8b, 9b | 5-Me-2-furyl | 112 | 96 | 99.5 (R)d |
| 24 | 8c, 9c | 3-Furyl | 72 | 99 | 99.4 (S) |
| 25 | 8d, 9d | 2-Thiophenyl | 86 | 95 | 99.2 (R) |
| 26 | 8e, 9e | NBoc-2-pyrryl | 21 | 99 | 99.5 (R) |
| 27 | 8f, 9f | NBoc-3-indolyl | 160 | 90 | 99.4 (S) |
| 28 | 10a, 11a | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
120 | 90 | 99.3 (S) |
| 29 | 10b, 11b | (E)-1-Penten-1-yl | 90 | 97 | 98.7 (S)d |
Hetarylic aldehydes 8a–f were also treated with nitromethane under these conditions (Table 1, entries 22–27). And again, the Henry products 9a–f were obtained in excellent yields (90–99%) and superb >99.0% ee, irrespective of the heterocycle (furyl, thiophenyl, or NBoc-pyrryl) and the substitution pattern.
The α,β-unsaturated aldehydes 10a and 10b solely afforded the 1,2-addition products 11a and 11b. The latter one is the only compound tested within this context that delivered less than 99.0% ee, namely 98.7%.
In all cases, the re-face of the aldehyde was attacked by the nitronate; the, in part, opposite absolute stereo descriptors in the products are a formal consequence of the CIP-notation.
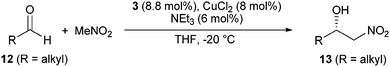
Aliphatic aldehydes 12 provided significantly lower enantioselectivities and yields under these conditions. Nonanal (12b), for example, delivered the Henry product 13b in unsatisfying 53% yield and 94.5% ee after 40 h. In order to compensate the lower reactivity, we raised the amount of catalyst to 8 mol% and the temperature to −20 °C, which afforded 13b in good 86% yield, but low 92.1% ee. Finally, a significant increase in the level of chirality transfer was observed by changing the copper salt from CuBr2 to CuCl2.14 Under these modified conditions, both, linear (12a–c) and α-branched (12d–g) aliphatic aldehydes provided the Henry products 13a–g in excellent 98.5–99.5% ee and >95% yield (Table 2).
| Entry | Compounds | R | Time (h) | Yieldb (%) | eec (%) (config.) |
|---|---|---|---|---|---|
| a Performed on a 1 mmol scale in THF (600 μL) and MeNO2 (600 μL ≈ 11 eq.). b Isolated yield. c Determined by HPLC analysis on a chiral phase; absolute configurations were established by comparison with literature data. | |||||
| 1 | 12a, 13a | nBu | 40 | 95 | 98.5 (S) |
| 2 | 12b, 13b | nOct | 60 | 97 | 98.6 (S) |
| 3 | 12c, 13c | PhCH2CH2 | 40 | 95 | 99.5 (S) |
| 4 | 12d, 13d | iPr | 44 | 96 | 99.1 (S) |
| 5 | 12e, 13e | cPent | 44 | 99 | 98.9 (S) |
| 6 | 12f, 13f | cHex | 44 | 99 | 99.4 (S) |
| 7 | 12g, 13g | tBu | 44 | 99 | 98.6 (S) |
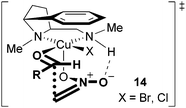
The stereochemical outcome of the Henry reactions can be explained via the transition state 14 (Fig. 2). As mentioned earlier, the aryl group of the chiral ligand 3 blocks the upper apical position at the Cu(II) ion, thus leaving three open coordination sites, two equatorial ones and one apical one. Based on the known model,10 the nitronate should bind apically for maximum activation, since its negative charge is less stabilised in this position by the copper ion. Of the two higher Lewis-acidic equatorial sites, the aldehyde should coordinate to the one next to the pyrrolidine moiety for two reasons: (i) this allows the sterically more demanding counter ion X to occupy the less congested position next to the aminomethyl group9b and (ii) with the weaker electron donating secondary amine opposite, the electrophilicity of the carbonyl group is increased thus facilitating a nucleophilic attack. Furthermore, the aldehyde must be oriented inwards in order to avoid severe steric repulsions with the chiral backbone. The C–C bond formation will presumably proceed via a six-membered, chair-shaped transition state, thus obviating repulsions between the nitronate-oxygen and the pyrrolidine N-methyl group.15 It might be possible that this arrangement receives some further stabilisation and rigidness by an intramolecular hydrogen bridge between the nitronate oxygen and the NH-proton of the chiral ligand. Thus, the steric and electronic properties of the diamine ligand apparently create close to perfect preconditions for the experimentally observed, almost exclusive re-face attack of the nitronate on the aldehyde carbonyl group.
In summary, the cis-5-phenyl substituted 2-aminomethylpyrrolidine 3, which is accessible in just a few steps from methyl Boc-L-pyroglutamate (4), was successfully utilized as the chiral ligand in CuBr2- and CuCl2-catalysed Henry reactions. Excellent isolated yields (>90%) and superb enantioselectivities (98.5–99.6% ee) were obtained with a wide variety of aromatic, heteroaromatic, vinylic and aliphatic aldehydes (36 examples). Further studies are ongoing.16
Financial support of the German research foundation (DFG) is gratefully acknowledged.
Notes and references
- Reviews: (a) F. A. Luzzio, Tetrahedron, 2001, 57, 915 CrossRef CAS; (b) The Nitro Group in Organic Synthesis, ed. N. Ono, Wiley-VCH, New York, 2001 Search PubMed.
- Reviews: (a) J. Boruwa, N. Gogoi, P. P. Saikia and N. C. Barua, Tetrahedron: Asymmetry, 2006, 17, 3315 CrossRef CAS PubMed; (b) C. Palomo, M. Oiarbide and A. Laso, Eur. J. Org. Chem., 2007, 2561 CrossRef CAS; (c) Y. Alvarez-Casao, E. Marques-Lopez and R. P. Herrera, Symmetry, 2011, 3, 220 CrossRef CAS PubMed; (d) G. Blay, V. Hernández-Olmos and J. R. Pedro, Synlett, 2011, 1195 CrossRef CAS PubMed; (e) G. Chelucci, Coord. Chem. Rev., 2013, 257, 1887 CrossRef CAS PubMed; (f) N. Ananthi and S. Velmathi, Indian J. Chem., Sect. B, 2013, 52, 87 Search PubMed.
- Selected examples: (a) H. Sasai, T. Suzuki, S. Arai, T. Arai and M. Shibasaki, J. Am. Chem. Soc., 1992, 114, 4418 CrossRef CAS; (b) T. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2009, 131, 13860 CrossRef CAS PubMed; (c) T. Nitabaru, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2012, 51, 1644 CrossRef CAS PubMed; (d) D. Sureshkumar, K. Hashimoto, N. Kumagai and M. Shibasaki, J. Org. Chem., 2013, 78, 11494 CrossRef CAS PubMed.
- Selected examples (except Cu-catalysis): (a) B. M. Trost and V. S. C. Yeh, Angew. Chem., Int. Ed., 2002, 41, 861 CrossRef CAS; (b) C. Palomo, M. Oiarbide and A. Laso, Angew. Chem., Int. Ed., 2005, 44, 3881 CrossRef CAS PubMed; (c) J. Park, K. Lang, K. A. Abboud and S. Hong, J. Am. Chem. Soc., 2008, 130, 16484 CrossRef CAS PubMed; (d) S. Liu and C. Wolf, Org. Lett., 2008, 10, 1831 CrossRef CAS PubMed; (e) R. Kowalczyk, P. Kwiatkowski, J. Skaržewski and J. Jurczak, J. Org. Chem., 2009, 74, 753 CrossRef CAS PubMed; (f) K. Lang, J. Park and S. Hong, Angew. Chem., Int. Ed., 2012, 51, 1620 CrossRef CAS PubMed.
- Cu-catalysed Henry reactions of nitromethane giving 99% ee with at least one aldehyde: (a) M. Bandini, F. Piccinelli, S. Tommasi, A. Umani-Ronchi and C. Ventrici, Chem. Commun., 2007, 616 RSC; (b) T. Arai, M. Watanabe and A. Yanagisawa, Org. Lett., 2007, 9, 3595 CrossRef CAS PubMed; (c) G. Zhang, E. Yashima and W.-D. Woggon, Adv. Synth. Catal., 2009, 351, 1255 CrossRef CAS; (d) W. Jin, X. Li, Y. Huang, F. Wu and B. Wan, Chem. – Eur. J., 2010, 16, 8259 CrossRef CAS PubMed; (e) G. Lai, F. Guo, Y. Zheng, Y. Fang, H. Song, K. Xu, S. Wang, Z. Zha and Z. Wang, Chem. – Eur. J., 2011, 17, 1114 CrossRef CAS PubMed; (f) A. T. Herrmann, S. R. Martinez and A. Zakarian, Org. Lett., 2011, 13, 3636 CrossRef CAS PubMed; (g) B. V. Subba Reddy and J. George, Tetrahedron: Asymmetry, 2011, 22, 1169 CrossRef CAS PubMed; (h) Y. Zhou, J. Dong, F. Zhang and Y. Gong, J. Org. Chem., 2011, 76, 588 CrossRef CAS PubMed; (i) W. Jin, X. Li and B. Wan, J. Org. Chem., 2011, 76, 484 CrossRef CAS PubMed; (j) R. I. Kureshy, A. Das, N. H. Khan, S. H. R. Abdi and H. C. Bajaj, ACS Catal., 2011, 1, 1529 CrossRef CAS; (k) Y. Wei, L. Yao, B. Zhang, W. He and S. Zhang, Tetrahedron, 2011, 67, 8552 CrossRef CAS PubMed; (l) L. Yao, Y. Wei, P. Wang, W. He and S. Zhang, Tetrahedron, 2012, 68, 9119 CrossRef CAS PubMed; (m) D.-D. Qin, W.-H. Lai, D. Hu, Z. Chen, A.-A. Wu, Y.-P. Ruan, Z.-H. Zhou and H.-B. Chen, Chem. – Eur. J., 2012, 18, 10515 CrossRef CAS PubMed; (n) K. Xu, G. Lai, Z. Zha, S. Pan, H. Chen and Z. Wang, Chem. – Eur. J., 2012, 18, 12357 CrossRef CAS PubMed; (o) A. Das, R. I. Kureshy, K. J. Prathap, M. K. Choudhary, G. V. S. Rao, N. H. Khan, S. H. R. Abdi and H. C. Bajaj, Appl. Catal., A, 2013, 459, 97 CrossRef CAS PubMed; (p) T. Deng and C. Cai, J. Fluorine Chem., 2013, 156, 183 CrossRef CAS PubMed; (q) R. Ćwiek, P. Niedziejko and Z. Kałuża, J. Org. Chem., 2014, 79, 1222 CrossRef PubMed.
- Selected recent examples of enantioselective Cu-catalysed Henry reactions: (a) G. Blay, V. Hernández-Olmos and J. R. Pedro, Org. Lett., 2010, 12, 3058 CrossRef CAS PubMed; (b) T. Arai, Y. Taneda and Y. Endo, Chem. Commun., 2010, 46, 7936 RSC; (c) L. Cheng, J. Dong, J. You, G. Gao and J. Lan, Chem. – Eur. J., 2010, 16, 6761 CrossRef CAS PubMed; (d) A. Chougnet, G. Zhang, K. Liu, D. Häussinger, A. Kägi, T. Allmendinger and W.-D. Woggon, Adv. Synth. Catal., 2011, 353, 1797 CrossRef CAS; (e) M. W. Leighty, B. Shen and J. N. Johnston, J. Am. Chem. Soc., 2012, 134, 15233 CrossRef CAS PubMed; (f) J. D. White and S. Shaw, Org. Lett., 2012, 14, 6270 CrossRef CAS PubMed; (g) Q. Dai, N. K. Rana and J. C.-G. Zhao, Org. Lett., 2013, 15, 2922 CrossRef CAS PubMed; (h) D.-D. Qin, W. Yu, J.-D. Zhou, Y.-C. Zhang, Y.-P. Ruan, Z.-H. Zhou and H.-B. Chen, Chem. – Eur. J., 2013, 19, 16541 CrossRef CAS PubMed.
- Selected recent examples: (a) D. Uraguchi, S. Nakamura and T. Ooi, Angew. Chem., Int. Ed., 2010, 49, 7562 CrossRef CAS PubMed; (b) K. Kanagaraj, P. Suresh and K. Pitchumani, Org. Lett., 2010, 12, 4070 CrossRef CAS PubMed; (c) Q. Dai, H. Huang and J. C.-G. Zhao, J. Org. Chem., 2013, 78, 4153 CrossRef CAS PubMed; (d) A. Quintavalla, M. Lombardo, S. P. Sanap and C. Trombini, Adv. Synth. Catal., 2013, 355, 938 CrossRef CAS; (e) S. Kitagaki, T. Ueda and C. Mukai, Chem. Commun., 2013, 49, 4030 RSC; (f) M. T. Corbett and J. S. Johnson, Angew. Chem., Int. Ed., 2014, 53, 255 CrossRef CAS PubMed.
- M. Gruber-Khadjawi, T. Purkarthofer, W. Skranc and H. Griengl, Adv. Synth. Catal., 2007, 349, 1445 CrossRef CAS.
- (a) M. Breuning, D. Hein, M. Steiner, V. H. Gessner and C. Strohmann, Chem. – Eur. J., 2009, 15, 12764 CrossRef CAS PubMed; (b) M. Breuning, M. Steiner, C. Mehler, A. Paasche and D. Hein, J. Org. Chem., 2009, 74, 1407 CrossRef CAS PubMed; (c) M. Breuning and D. Hein, Eur. J. Org. Chem., 2013, 7575 CrossRef CAS.
- D. A. Evans, D. Seidel, M. Rueping, H. W. Lam, J. T. Shaw and C. W. Downey, J. Am. Chem. Soc., 2003, 125, 12692 CrossRef CAS PubMed.
- Enantioselective, Cu-catalysed Henry reactions using proline-derived ligands: (a) B. V. Subba Reddy, S. Madhusudana Reddy, S. Manisha and C. Madan, Tetrahedron: Asymmetry, 2011, 22, 530 CrossRef PubMed; (b) D. Lu, Y. Zhou, Y. Li, S. Yan and Y. Gong, J. Org. Chem., 2011, 76, 8869 CrossRef CAS PubMed; (c) B. Ni and J. He, Tetrahedron Lett., 2013, 54, 462 CrossRef CAS PubMed; (d) Y. Zhou, Y. Zhu, S. Yan and Y. Gong, Angew. Chem., Int. Ed., 2013, 52, 10265 CrossRef CAS PubMed; (e) H. Yuan, J. Hu and Y. Gong, Tetrahedron: Asymmetry, 2013, 24, 699 CrossRef CAS PubMed; (f) H. A. Sema, G. Bez and S. Karmakar, Appl. Organomet. Chem., 2014, 28, 290 CrossRef; (g) Ref. 5e, h and n .
- Details about the diamine screening and the optimization of the reactions conditions as well as further mechanistic investigations will be reported elsewhere.
- Synthesis of related compounds: (a) M.-C. Fournie-Zaluski, P. Coric, V. Thery, W. Gonzalez, H. Meudal, S. Turcaud, J.-B. Michel and B. P. Roques, J. Med. Chem., 1996, 39, 2594 CrossRef CAS PubMed; (b) A. Momotake, H. Togo and M. Yokoyama, J. Chem. Soc., Perkin Trans. 1, 1999, 1193 RSC; (c) A. C. Rudolph, R. Machauer and S. F. Martin, Tetrahedron Lett., 2004, 45, 4895 CrossRef CAS PubMed.
- A similar effect on the ee was not observed with aromatic aldehydes.
- A boat-type transition state cannot fully be excluded, but seems less likely.
- Initial studies on Henry reactions with other nitroalkanes revealed acceptable to good diastereoselectivities and excellent enantioselectivities in the major syn-diastereomer. The reaction of benzaldehyde (6a) with nitropropane, for example, afforded the corresponding β-nitro alcohol in 99% yield with a syn/anti ratio of 79
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and 98% ee in the major diastereomer.
21 and 98% ee in the major diastereomer.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, HPLC- and NMR spectra. See DOI: 10.1039/c4cc02429j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |