Open Access Article
Open Access ArticleBoron-nitride and aluminum-nitride “Pringles” and flapping motion†
Wei
Fa
*ab,
Shuang
Chen
b and
Xiao Cheng
Zeng
*b
aNational Laboratory of Solid State Microstructures and Department of Physics, Nanjing University, Nanjing, 210093, China. E-mail: wfa@nju.edu.cn
bDepartment of Chemistry, University of Nebraska-Lincoln, Lincoln, Nebraska 68588, USA. E-mail: xzeng1@unl.edu
First published on 19th May 2014
Abstract
Motivated by the recent successful synthesis of a new nanocarbon, namely, a warped, double-concave graphene “Pringle” (Nat. Chem., 2013, 5, 739), we investigate properties of warped boron-nitride (BN) and aluminum-nitride (AlN) analogues, i.e., the non-planar B40N40H30 and Al40N40H30 “Pringles” using density functional theory (DFT) calculations. Particular attention is placed on the effect of non-hexagonal rings on the stability and physical properties of BN and AlN Pringles. We find that the warped BN and AlN Pringles with one pentagon and five heptagons are stable without imaginary frequencies. Both the warped B40N40H30 and Al40N40H30 Pringles are expected to be flexible in solution as both can periodically change their shape in a dynamic “flapping” fashion due to their much lower activation barrier of racemization compared to that of the C80H30 counterpart. Since the warped B40N40H30 possesses a smaller HOMO–LUMO gap than the planar B39N39H30, it is expected that incorporating non-hexagonal ring defects by design can be an effective way to modify electronic properties of BN-based nanoplates.
The discovery of the C60 fullerene,1 carbon nanotubes2 as well as monolayer graphene3 has attracted tremendous interest in seeking new members in the nanocarbon family as well as low-dimensional nanomaterials of carbon analogues. Indeed, a variety of structure analogues of nanocarbons without containing carbon elements has been synthesized in the laboratory. Well-known examples are the boron-nitride (BN) nanomaterials which can be viewed as isoelectronic “cousins” to many nanocarbon allotropes.4–10 Unlike the covalent C–C bonds, the partially ionic B–N bonds can significantly affect geometric and electronic structures of BN nanomaterials. For example, a BN monolayer is a wide direct bandgap semiconductor while monolayer graphene is a semimetal with a zero bandgap.11–14 Note however that BN cages are structurally dissimilar to carbon fullerenes. This is because BN clusters with perfect BNBN alternation are energetically preferred due to less strain energy and aromatic destabilization.15,16
It is known that the presence of defects may induce notable changes in nanostructures, thereby modifying nanomaterials' physical properties. As an example, line defects in the hexagonal BN (h-BN) monolayer, characterized by boundaries between fcc domains and a small population of hcp domains, have been observed during the growth of h-BN on the Ni(111) surface.17 The existence of an extended line defect in a BN sheet presents a way to modify electronic or magnetic properties of the BN sheet for potential applications in nanoelectronics and spintronics. A recent theoretical study of line-defect-containing BN sheets, nanoribbons, and single-walled BN nanotubes shows that the bandgaps can be changed by the pentagon–octagon–pentagon line defects created by inserting B2, N2, or C2 dimers.18 Yamijala and Pati found that electronic and magnetic properties of a BN nanoribbon can be modified by adding a number of (odd or even) pentagon–heptagon line defects at the ribbon edges.19 These studies suggest that controlled topological (non-hexagonal rings) defects can be useful to modulate electronic properties of BN nanostructures.
Very recently, a new carbon nanostructure belonging to the nanocarbon family, i.e., the first non-planar nanographene, has been reported.20 Kawasumi et al. successfully synthesized a grossly warped nanographene C80H30 having twenty-six polygons, among which five are heptagons and one is pentagon. The introduction of five heptagons not only causes the nanographene to warp but also alters its electronic and optical properties. This warped nanographene exhibits many unique features such as a facile bowl-to-bowl inversion of the central corannulene, a unique racemization pathway, as well as a larger gap (3.06 eV) between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). In view of many structural similarities between BN and carbon nanostructures, it is expected that the embedding of non-hexagonal rings into a BN nanoplate would provide a new member of BN nanostructures as well. Besides BN, we also investigate a warped aluminium-nitride (AlN) nanoplate for the purpose of comparison. AlN nanomaterials are often used in deep ultraviolet optoelectronics, and as building blocks in new nanomaterials.21–24
We carry out a series of density functional theory (DFT) calculations to investigate the structural, electronic, and optical properties of the warped BN and AlN nanoplates. We show that the grossly warped nanostructures of B40N40H30 and Al40N40H30 with multiple odd-membered-ring defects are locally stable and their electronic and optical properties can be modified by the non-hexagonal ring defects. The warped B40N40H30 exhibits a markedly reduced HOMO–LUMO gap and red-shifted optical absorption spectra compared to the planar B39N39H30 with perfect BNBN alternation.
Geometry optimizations are performed using the B3LYP functional and the 6-31G(d) basis set. Computational details and validation of the computational methods are given in the ESI† (Tables S1 and S2 and Fig. S1). The initial structures are constructed from the 26-ring C80H30 as a template. With five heptagons and one pentagon added in the nanoplates, the B–B, Al–Al, or N–N bonds are formed at the pentagonal and heptagonal sites. Thus, there are at least six homonuclear bonds in the warped structure. The other sites exhibit alternate B–N bonds.
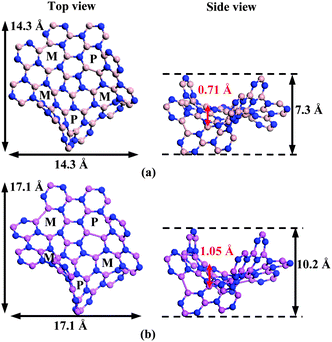
First, various isomers of the BN and AlN nanoplates are examined (see ESI,† Fig. S2–S4) and the lowest-energy B40N40H30 and Al40N40H30 isomers are identified (see Fig. 1). Both lowest-energy nanostructures exhibit similar configurations with maximum 19 hexagons in an alternating BNBN (or AlNAlN) sequence. Other higher-energy isomers typically have less number of hexagons with BNBN (or AlNAlN) alternation. More specifically, the lowest-energy B40N40H30 is 3.85 eV lower in energy than the isomer with 18 BNBN alternative hexagons, which can be viewed as exchanging a pair of B and N atoms at the edge of the lowest-energy isomer B40N40H30. This result confirms the previous theoretical prediction that the B–B and N–N bonds should be avoided as much as possible in BN clusters as they would result in much less stable isomers.15 Like the warped C80H30 with enantiomers of MPMPM and PMPMP configurations, the presence of five helical hexa[7]circulene moieties,25 each with M or P chirality around the heptagon, also renders the lowest-energy structures having an isoenergetic enantiomer of PMPMP (see below). Computed vibrational spectra of the warped B40N40H30 and Al40N40H30 have a frequency range of 10.6–3622.2 and 3.5–3561.7 cm−1, respectively, and the spectra exhibit a strong peak at 1440 and 930 cm−1, respectively. These strong peaks can be used as a fingerprint to determine the warped nanostructures in future experiments. Note that the highest vibrational frequency of the warped B40N40H30 is higher than that of the Al40N40H30 or C80H30 (3242.3 cm−1), reflecting stronger B–N bonds than Al–N bonds and C–C bonds (see ESI,† Fig. S5).
We find that the warped B40N40H30 and Al40N40H30 not only can flip back and forth between two different conformers through bowl-to-bowl inversion, but also change between two enantiomers through a racemization pathway (see Fig. 2 or ESI,† Fig. S6 for an enlarged view). The “flipping” behavior is due to the presence of the central pentagon defect while the five pentagons induce negative curvature. For the bowl-to-bowl inversion as illustrated in Fig. 2 (MPMPM ⇔ TSflip ⇔ MPMPM), the computed bowl inversion energy of the C80H30 is 1.7 kcal mol−1, in agreement with the result of ref. 20. However, both B40N40H30 and Al40N40H30 exhibit a deeper bowl structure (see Fig. 1) compared to the warped C80H30 (with bowl depth 0.37 Å). Hence, for the B40N40H30 and Al40N40H30 nanoplates, the activation energy of the bowl inversion is 27.9 and 23.2 kcal mol−1, respectively, much higher than that of C80H30. The B40N40H30 nanoplate possesses the highest bowl inversion energy (27.9 kcal mol−1) due also to the strong B–N bonds. A recent study shows that the bowl inversion energy of a chiral nitrogen-doped carbon-bowl reaches an extraordinarily high value of 42.2 kcal mol−1 due largely to the strong C–N bonds.26
The computed energy barrier for the racemization of the B40N40H30, as shown in Fig. 2 (MPMPM ⇔ TSrac ⇔ PMPMP), is merely 4.3 kcal mol−1, which is much lower than that (18.9 kcal mol−1) for the C80H30 nanographene. To simulate the racemization process of the B40N40H30 nanoplate, we perform a Born–Oppenheimer molecular dynamics simulation (see ESI,† Movie S1) to demonstrate the iterative racemization transitions between the MPMPM and PMPMP enantiomers. For the Al40N40H30 nanoplate, the computed activation energy of racemization is 6.7 kcal mol−1. Hence, both the B40N40H30 and Al40N40H30 nanoplates are expected to exhibit “flapping” motion periodically in solutions.
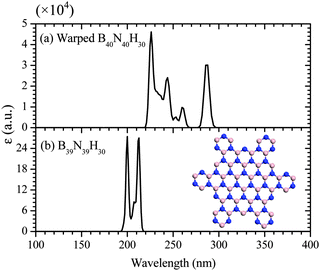
As expected, the addition of non-hexagonal rings modifies electronic and optical properties of the B40N40H30 and Al40N40H30 Pringles. For example, data for the comparison between the warped B40N40H30 and a planar and fully BNBN alternating B39N39H30 nanoplate (see the inset image in Fig. 3(b)) clearly show that the HOMO–LUMO gap can be appreciably reduced via embedding non-hexagonal rings into the BN nanoplate. The HOMO-energy (EHOMO = −5.777 eV) of the B40N40H30 is shifted upward compared to that of the planar B39N39H30 (EHOMO = −6.487 eV), while the LUMO is shifted downward (ELUMO = −0.763 versus −0.016 eV), leading to a narrower HOMO–LUMO gap (5.01 eV) for the warped structure than that (6.47 eV) of the planar B39N39H30. Based on this result, we expect that the introduction of non-hexagonal rings into the BN sheet, a wide-gap semiconductor, can also reduce the bandgap of the BN sheet.
The effect of the non-hexagonal rings on optical properties is illustrated in Fig. 3, where the computed optical absorption spectra of the warped B40N40H30 and the planar B39N39H30 are shown. A major difference between the two spectra is the peaks in the ultraviolet region. The planar B39N39H30 exhibits two sharp peaks at 200 and 213 nm, respectively; the second peak has a shoulder at 207 nm. The spectrum of the warped B40N40H30 exhibits richer features with at least four well-resolved peaks located at 226, 244, 260, and 288 nm, respectively. Compared to the feature peaks of the planar B39N39H30, the peaks of warped B40N40H30 can be viewed as red-shifted due in part to the narrower HOMO–LUMO gap of the warped structure. These features can be used to differentiate the warped and planar BN nanostructures.
In summary, we investigate structural and electronic properties of warped B40N40H30 and Al40N40H30 nanoplates or Pringles. Both B40N40H30 and Al40N40H30 nanoplates are local minima on the potential energy surfaces without imaginary frequencies. Compared to the C80H30 counterpart, the B40N40H30 and Al40N40H30 nanoplates have much higher bowl inversion energy due to their deeper bowl depth and relatively stronger chemical bonds B–N (or Al–N) bonds. More interestingly, the B40N40H30 and Al40N40H30 nanoplates are expected to exhibit “flapping” motion in solution due to the much lower energy barrier of the racemization compared to that of the C80H30 counterpart. Finally, embedding non-hexagonal rings in BN nanoplates can reduce the HOMO–LUMO gap. Such a bandgap-reduction by introduction of non-hexagonal rings can exploit electronic properties of BN nanostructures for nanoelectronic applications.
WF acknowledges the State Scholarship Fund provided by the China Scholarship Council through No. 201308320156. XCZ is supported by ARL (Grant No. W911NF1020099), NSF (Grant No. DMR-0820521), and UNL Holland Computing Center, and a grant from USTC for (1000 Talents Plan) summer research.
Notes and references
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS
.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS
.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Girgorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed
.
- N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966–967 CrossRef CAS PubMed
.
- W. Mickelson, S. Aloni, W. Q. Han, J. Cumings and A. Zettl, Science, 2003, 300, 467–469 CrossRef CAS PubMed
.
- A. Loiseau, F. Willaime, N. Demoncy, G. Hug and H. Pascard, Phys. Rev. Lett., 1996, 76, 4737–4740 CrossRef CAS
.
- X. Xia, D. A. Jelski, J. R. Bowser and T. F. George, J. Am. Chem. Soc., 1992, 114, 6493–6496 CrossRef CAS
.
- F. Jensen, Chem. Phys. Lett., 1993, 209, 417–422 CrossRef CAS
.
- D. L. Strout, J. Phys. Chem. A, 2001, 105, 261–263 CrossRef CAS
.
- M. Monajjemi and J. E. Boggs, J. Phys. Chem. A, 2013, 117, 1670–1684 CrossRef CAS PubMed
.
- Y. B. Zhang, Y. W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201–204 CrossRef CAS PubMed
.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed
.
- A. Nag, K. Raidongia, K. P. S. S. Hembram, R. Datta, U. V. Waghmare and C. N. R. Rao, ACS Nano, 2010, 4, 1539–1544 CrossRef CAS PubMed
.
- D. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang and C. Zhi, ACS Nano, 2010, 4, 2979–2993 CrossRef CAS PubMed
.
- F. Jensen and H. Toftlund, Chem. Phys. Lett., 1993, 201, 89–96 CrossRef CAS
.
- H. Y. Zhu, T. G. Schmalz and D. J. Klein, Int. J. Quantum Chem., 1997, 63, 393–401 CrossRef CAS
.
- W. Auwärter, M. Muntwiler, J. Osterwalder and T. Greber, Surf. Sci., 2003, 545, L735–L740 CrossRef PubMed
.
- X. Li, X. Wu, X. C. Zeng and J. Yang, ACS Nano, 2012, 6, 4104–4112 CrossRef CAS PubMed
.
- S. S. Yamijala and S. K. Pati, J. Phys. Chem. C, 2013, 117, 3580–3594 CAS
.
- K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott and K. Itami, Nat. Chem., 2013, 5, 739–744 CrossRef CAS PubMed
.
- C. Liu, Z. Hu, Q. Wu, X. Wang, Y. Chen, H. Sang, J. Zhu, S. Deng and N. Xu, J. Am. Chem. Soc., 2005, 127, 1318–1322 CrossRef CAS PubMed
.
- X. H. Ji, S. P. Lau, S. F. Yu, H. Y. Yang, T. S. Herng, A. Sedhain, J. Y. Lin, H. X. Jiang, K. S. Teng and J. S. Chen, Appl. Phys. Lett., 2007, 90, 193118 CrossRef PubMed
.
- H. Wang, Z. Xie, Y. Wang, W. Yang, Q. Zeng, F. Xing and L. An, Nanotechnology, 2009, 20, 025611 CrossRef PubMed
.
- Y. Mei, D. J. Thurmer, C. Deneke, S. Kiravittaya, Y. F. Chen, A. Dadgar, F. Bertram, B. Baster, A. Krost, J. Christen, T. Reindl, M. Stoffel, E. Coric and O. G. Schmidt, ACS Nano, 2009, 3, 1663–1668 CrossRef CAS PubMed
.
- P. J. Jessup and J. A. Reiss, Aust. J. Chem., 1976, 29, 173–176 CrossRef CAS
.
- Q. Tan, S. Higashibayashi, S. Karanjit and H. Sakurai, Nat. Commun., 2012, 3, 891 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Computational details, different nanoplate isomers, vibrational analysis of the optimized structures, Cartesian coordinates, and Born–Oppenheimer molecular dynamics simulation of the racemization process of the B40N40H30 nanoplate. See DOI: 10.1039/c4cc02294g |
| This journal is © The Royal Society of Chemistry 2014 |