 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective ratiometric detection of H2O2 in water and in living cells with boronobenzo[b]quinolizinium derivatives†
Roberta
Bortolozzi
a,
Sebastian von
Gradowski
b,
Heiko
Ihmels
*b,
Katy
Schäfer
b and
Giampietro
Viola‡
a
aDipartimento di Salute della Donna e del Bambino, Laboratorio di Oncoematologia, University of Padova, via Giustiniani 2, I-35128 Padova, Italy
bDepartment Chemie - Biologie, Universität Siegen, Adolf-Reichwein-Str. 2, 57068 Siegen, Germany. E-mail: ihmels@chemie.uni-siegen.de
First published on 9th June 2014
Abstract
Boronobenzo[b]quinolizinium derivatives exhibit several favorable properties for the fluorimetric detection of hydrogen peroxide, namely quantitative transformation to a product whose emission maximum is well separated from the one of the substrate, water solubility, and the ability to operate in living cells.
Reactive oxygen species (ROS), such as e.g. hydrogen peroxide, singlet oxygen, the hydroxyl radical, the superoxide anion, peroxynitrite, or ozone,1 have fundamental functions in health and disease related processes.2 Specifically, increased concentrations of H2O2 may cause oxidative damage of cellular proteins, ageing and diseases such as cancer or diabetes.3 Therefore, the detection of H2O2 and other ROS is a challenging and important task in biological and chemical research. Along these lines, fluorescent probes have been established as sensitive and selective tools for the detection of H2O2.4a Fluorescent light-up probes offer one approach to respond to H2O2 by an emission increase.5 These intensity-based probes may have practical advantages; however, they suffer from the fact that the emission intensity is also effectively influenced by other internal and external factors.4b A complementary approach towards fluorimetric detection of ROS is based on ratiometric probes operating at two different detection wavelengths that enable an accurate internal calibration.6 In this context aryl boronates were identified as probe molecules whose selective reaction with H2O2 may be employed for the development of ratiometric fluorescent probes.7 In general the transformation of the boronic acid (ester) group into an hydroxy functionality results in a significant change of the absorption and emission properties of the fluorophore thus enabling a ratiometric analysis. Based on this principle, several ratiometric H2O2-sensitive probes were developed.8 Nevertheless, the development of novel ratiometric fluorescent chemosensors for the detection of H2O2 is still an important goal, especially those with sufficient water solubility. The latter is an essential requirement for studies in biological media. In this context we have established the benzo[b]quinolizinium as a versatile, water-soluble fluorophore for fluorimetric detections.9 In addition, we have discovered that the hydroxy-substituted benzo[b]quinolizinium derivative 2b has fluorosolvatochromic properties with a large redshift of the emission band in water (600 nm).10 At the same time, the 9-boronobenzo[b]quinolizinium (1a) exhibits the same emission bands as the parent compound (417 nm).11 Considering this large difference of emission color of borono- and hydroxy-substituted derivatives we proposed that the formation of the latter by the reaction of H2O2 with boronobenzo[b]quinolizinium derivatives may be monitored by a drastic shift of the emission wavelength. Meanwhile, it was shown that resembling quinolinium-type boronic acid esters may indeed be used for that purpose.8a Herein, we will demonstrate that the boronic acids and ester derivatives 1a–d may be generally employed as ratiometric fluorescence probes for the detection of H2O2 in aqueous solution and in living cells (Scheme 1).
The benzo[b]quinolizinium derivatives 1a,b and 2a,b were synthesized by the cyclodehydration method,12 and the two acid derivatives 1a,b were transformed to the corresponding pinacol-boronates 1c,d (cf. ESI†). Known compounds (1a, 2a,b) were identified by comparison with literature data.10,11,12b All new compounds (1b–d) were characterized by 1H-NMR and 13C-NMR spectroscopy, mass spectrometry and elemental analysis (cf. ESI†). The absorption spectra of the derivatives 1a–d in phosphate buffer solution reveal the characteristic long-wavelength absorption band of the benzo[b]quinolizinium chromophore13 with a maximum at 380 nm (1a,c) and 377 nm (1b,d) (cf. ESI†). The hydroxy-substituted derivatives have absorption maxima at 387 nm (2a) and 379 nm (2b),10 along with a weak, very broad redshifted charge transfer (CT) band at ca. 450 nm.10 The emission properties of the boronic acid derivatives 1a–d resemble the ones of the parent benzo[b]quinolizinium (λfl = 410–415 nm; Φfl = 0.03–012). In contrast, the fluorescence maxima of the hydroxybenzo[b]quinolizinium derivatives are significantly redshifted with maxima at 527 nm (2a) and 600 nm (2b).10
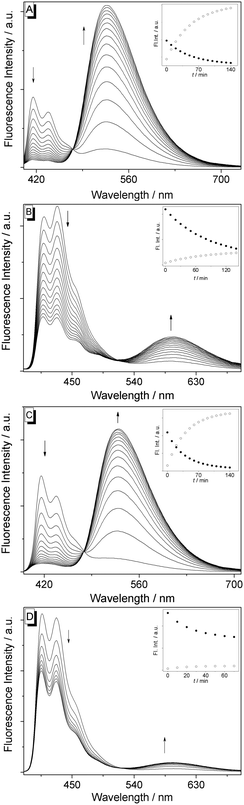
The reaction of the derivatives 1a–d with H2O2 in buffer solution produced the corresponding hydroxybenzo[b]quinolizinium derivatives 2a and 2b (Scheme 1). Most notably, in the case of 1a–d the 1H-NMR-spectroscopic analysis of the reaction revealed a quantitative transformation with no detectable side products (cf. ESI†). The reaction of compounds 1a–d with H2O2 in buffer solution was also monitored by emission spectroscopy (Fig. 1). The progress of the reaction is clearly indicated by a continuous decrease of the intensity of the emission maximum of compounds 1a–1d (415 or 417 nm) that is accompanied by the simultaneous increase of a new redshifted emission band at 527 nm and 600 nm, respectively. The latter emission bands are identical to the ones obtained with authentic samples of the hydroxy-substituted derivatives 2a,b. The ratios of the intensities of the emission maxima increase by a factor of 17 (1a) and 0.7 (1b) at 140 min after addition of H2O2, as quantified by the term Ired/Iblue. Control experiments showed that the formation of 2a and 2b does not take place in buffer solution in the absence of H2O2. The results indicate full conversion of the substrates 1a,b to the products 2a,b within 140 min (1a) and 160 min (1b). The limit of detection was determined to be 3.0 μM for 1a and 5.9 μM for 1c (cf. ESI†), which is comparable to that of established probes.5b,8a,c The reaction rate was analyzed considering pseudo first-order kinetics according to an established protocol.8c By this method, rate constants of kobs = 4.3 × 10−4 s−1 (1a) and kobs = 2.9 × 10−4 s−1 (1b) were obtained (cf. ESI†). To assess the selectivity of the oxidation reaction of compounds 1a–d they were also treated with other oxidants, namely O2˙−, 1O2, ClO−, NO˙, peroxides ROO˙, HO˙, and ONO2−. The reaction was monitored by fluorescence spectroscopy, and the ratiometric analysis, Ired/Iblue, was used to determine the progress of the reaction (Fig. 2). Notably, the compounds 1a and 1c are efficiently oxidized by H2O2, whereas the other oxidants only induce the formation of the oxidation product 2a to marginal or moderate (O2˙−, HO˙, ONO2−) extent under resembling reaction conditions. A similar trend was observed for the derivatives 1b and 1d, however, the selectivity is less pronounced, and the ratio of Ired/Iblue is smaller because of the lower emission intensity of the reaction product 2b.
The ability of the derivatives 1c and 1d to detect varying levels of H2O2 in living cells was assessed. For this purpose, we used HeLa cell lines, i.e. an adenocarcinoma that grows as monolayer, and Jurkat cells, i.e. leukemia cell lines that grow in suspension. In preliminary experiments the cellular uptake by compound 1c was evaluated by flow cytometry taking advantage of the intrinsic fluorescence of the compound (cf. ESI†). The cellular uptake was rapid and reached a plateau after 15–20 min of incubation in Jurkat cells and after 1 h in HeLa cells suggesting an efficient delivery of the compound inside the cells. After 1 h of incubation 1c and 1d emit at 450 nm (FL9 channel), while no emission was detectable at 540 nm (FL10 channel) (cf. ESI†). To evaluate the ability of derivatives 1c and 1d to respond to H2O2 in a living cell, HeLa cells were treated with H2O2 (100 μM) after the incubation with 1c and 1d, and the relative fluorescence intensities of the cells were analyzed by flow cytometry (Fig. 3). After 10 min the fluorescence intensity of 1c at 450 nm decreased, and a signal at 540 nm developed (Fig. 3A and B). This development of emission signals may be followed also by a ratiometric analysis (cf. ESI†). In the case of 1d, however, only the signal at 450 nm disappeared, but no redshifted emission was observed.
Furthermore, we evaluated if 1c could be useful to detect physiological generation of H2O2 under pharmacological induction. Thus, both HeLa and Jurkat cells were treated with staurosporine, that is known to induce apoptosis through mitochondrial membrane depolarization. The latter has been associated with mitochondrial production of reactive oxygen species (ROS), in particular H2O2.14 HeLa and Jurkat cells were treated with the drug for 12 h and afterwards incubated with 1c for 30 min. Subsequent analysis by flow cytometry showed a significant increase of the population of cells that emit at 540 nm in both cell lines (Fig. 4), thus demonstrating the capability of compound 1c to indicate pharmacologically stimulated formation of intracellular H2O2.
 | ||
| Fig. 4 Flow cytometric analysis of HeLa (A) and Jurkat cells (B) treated for 24 h with staurosporine (1 μM) and subsequently incubated with 1c for 20 min. | ||
In summary we have demonstrated that boronobenzo[b]quinolizinium derivatives exhibit several favorable properties for fluorimetric detection of hydrogen peroxide, namely water solubility, quantitative transformation to a product whose emission maximum is well separated from the one of the substrate thus allowing ratiometric analysis, and the general ability to operate in living cells. With respect to a relationship between function and structure, it appears that benzo[b]quinolizinium fluorophores, in which the ROS-sensitive borono-functionality as well as the eventually formed hydroxy functionality are linearly conjugated with the pyridinium unit, provide the more effective structural platform for fluorimetric detection as compared to the cross-conjugated system. Specifically, the 9-substituted derivatives 1a and 1c exhibit a more pronounced light-up effect and a higher selectivity towards H2O2 than the 8-substituted ones. It should be noted that these probes may be easily varied by the attachment of other functionalities R1 that are transformed to the strongly solvatochromic hydroxy-substituted derivative 2a.
Generous support by the Deutsche Forschungsgemeinschaft is gratefully acknowledged.
Notes and references
- M. P. Fink, Curr. Opin. Crit. Care, 2002, 8, 6–11 CrossRef PubMed.
- (a) P. Niethammer, C. Grabher, A. T. Look and T. J. Mitchison, Nature, 2009, 459, 996–999 CrossRef CAS PubMed; (b) G. Groeger, C. Quiney and T. G. Cotter, Antioxid. Redox Signaling, 2009, 11, 2655–2671 CrossRef CAS PubMed; (c) E. A. Veal, A. M. Day and B. A. Morgan, Mol. Cell, 2007, 26, 1–14 CrossRef CAS PubMed; (d) J. Li, M. Stouffs, L. Serrander, B. Banfi, E. Bettiol, Y. Charnay, K. Steger, K. H. Krause and M. E. Jaconi, Mol. Biol. Cell, 2006, 17, 3978–3988 CrossRef CAS PubMed; (e) M. Ushio-Fukai, Cardiovasc. Res., 2006, 71, 226–235 CrossRef CAS PubMed; (f) E. R. Stadtman, Free Radical Res., 2006, 40, 1250–1258 CrossRef CAS PubMed.
- D. Hernández-García, C. D. Wood, S. Castro-Obregón and L. Covarrubias, Free Radical Biol. Med., 2010, 49, 130–143 CrossRef PubMed.
- (a) C. C. Winterbourn, Biochim. Biophys. Acta, 2014, 1840, 730–738 CrossRef CAS PubMed; (b) Y. H. A. Liu and X. B. Liao, Curr. Org. Chem., 2013, 17, 654–669 CrossRef CAS.
- See e.g. (a) M. Kaur, D. S. Yang, K. Choi, M. J. Cho and D. H. Choi, Dyes Pigm., 2014, 100, 118–126 CrossRef CAS PubMed; (b) K. B. Daniel, A. Agrawal, M. Manchester and S. M. Cohen, ChemBioChem, 2013, 14, 593–598 CrossRef CAS PubMed; (c) L. Yuan, W. Lin, Y. Xie, B. Chen and S. Zhu, J. Am. Chem. Soc., 2012, 134, 1305–1315 CrossRef CAS PubMed; (d) Y. Ahn, K. E. Fairfull-Smith, B. J. Morrow, V. Lussini, B. Kim, M. V. Bondar, S. E. Bottle and K. D. Belfield, J. Am. Chem. Soc., 2012, 134, 4721–4730 CrossRef PubMed; (e) L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed; (f) N. Karton-Lifshin, E. Segal, L. Omer, M. Portnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960–10965 CrossRef CAS PubMed.
- (a) C.-Y. Chen and C.-T. Chen, Chem. – Eur. J., 2013, 19, 16050–16057 CrossRef CAS PubMed; (b) A. R. Lippert, G. C. V. De Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS PubMed; (c) A. E. Albers, V. S. Okreglak and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9640–9641 CrossRef CAS PubMed.
- (a) Y. H. A. Liu and X. B. Liao, Curr. Org. Chem., 2013, 17, 654–669 CrossRef CAS; (b) Z. Q. Guo, I. Shin and J. Yoon, Chem. Commun., 2012, 48, 5956–5967 RSC; (c) For mechanism, see: H. G. Kuivila and A. G. Armour, J. Am. Chem. Soc., 1957, 79, 5659 CrossRef CAS.
- (a) S. W. Lee, H.-W. Rhee, Y.-T. Chang and J.-I. Hong, Chem. – Eur. J., 2013, 19, 14791–14794 CrossRef CAS PubMed; (b) G. P. Li, D. J. Zhu, Q. Liu, L. Xue and H. Jiang, Org. Lett., 2013, 15, 924–927 CrossRef CAS PubMed; (c) G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518–3520 RSC; (d) D. Srikun, E. W. Miller, D. W. Dornaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed; (e) A. E. Albers, V. S. Okreglak and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9640–9641 CrossRef CAS PubMed.
- (a) R. Bortolozzi, H. Ihmels, L. Thomas, M. Tian and G. Viola, Chem. – Eur. J., 2013, 19, 8736–8741 CrossRef CAS PubMed; (b) M. Tian, H. Ihmels and S. T. Ye, Org. Biomol. Chem., 2012, 10, 3010–3018 RSC; (c) M. Tian, H. Ihmels and K. Benner, Chem. Commun., 2010, 46, 5719–5721 RSC; (d) M. Tian and H. Ihmels, Chem. Commun., 2009, 3175–3177 RSC; (e) A. Bergen, A. Granzhan and H. Ihmels, Photochem. Photobiol. Sci., 2008, 7, 405–407 RSC.
- H. Ihmels and K. Schäfer, Photochem. Photobiol. Sci., 2009, 8, 309–311 CAS.
- M. Tian and H. Ihmels, Synthesis, 2009, 4226–4234 CAS.
- (a) C. K. Bradsher, Chem. Rev., 1946, 46, 447–499 CrossRef; (b) C. K. Bradsher and J. H. Jones, J. Am. Chem. Soc., 1957, 79, 6033–6034 CrossRef CAS.
- S. U. D. Saraf, Heterocycles, 1981, 16, 987–1007 CrossRef CAS.
- (a) H. Nohl, L. Gille and K. Staniek, Biochem. Pharmacol., 2005, 69, 719–723 CrossRef CAS PubMed; (b) J. Cai and D. P. Jones, J. Biol. Chem., 1998, 273, 11401–11404 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, full characterization and NMR spectra, spectroscopic studies. See DOI: 10.1039/c4cc02283a |
| ‡ Author names in alphabetical order that does not reflect the specific contribution of each author. |
| This journal is © The Royal Society of Chemistry 2014 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells for each analysis.
000 cells for each analysis.