Open Access Article
Open Access ArticleBoron–nitrogen substituted perylene obtained through photocyclisation†
Matthias
Müller
a,
Stefan
Behnle
a,
Cäcilia
Maichle-Mössmer
b and
Holger F.
Bettinger
*a
aInstitut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: Holger.Bettinger@uni-tuebingen.de; Fax: +49-7071/29-5244; Tel: +49-7071/29-72072
bInstitut für Anorganische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany
First published on 6th June 2014
Abstract
A BN substituted hexabenzotriphenylene (B3N3) closes one C–C-bond upon irradiation with light of 280–400 nm in the presence of iodine to yield a phenanthrene annelated B3N3 tribenzoperylene. Upon hydrolysis a B2N2 dibenzoperylene is obtained.
Atom-precise build-up of doped graphene (sub)structures is one of the challenging synthetic problems of these days.1 These polycyclic aromatic hydrocarbons have astonishing properties that could show superior performance1a,2 compared to their all-carbon analogues in industrial applications.3 To tune electronic properties, or to be more accurate the HOMO–LUMO gap,4 synthetic tools and handles to implement changes have to be available. While all-carbon PAHs only offer the opportunity for alteration at the periphery, substitution of carbon atoms by other suitable elements may provide a means to improve electronics without significantly changing other parameters like bond length5 and crystal packing.6 In this regard a long known concept is the isoelectronic substitution of CC units by BN moieties, which only alters electronics without changing geometrical properties.7 But although several aromatic hydrocarbons with incorporated BN-units are known, there are only very few methods available for their synthesis. While some synthetic methods are restricted to one special BN-PAH without the possibility of extending their scope,8 others have proven to be of conceptual relevance as they are useful for a broader range of boron nitrogen aromatics. The oldest known concept to produce BN-PAHs involves hydroboration of suitable alkenes in the key step.8a,9 Another strategy introduced by Piers et al. relies on the aromatization of boron containing heterocycles by the extrusion of trimethylsilylchloride when reacted with azaheterocycles (pyridine, pyridazine,…).2,6,10 A route that is mainly used for the synthesis of different 1,2-dihydro-1,2-azaborines utilizes ring closing metathesis.11 Introduction of BN units into aromatic hydrocarbons can also be accomplished by Lewis acid (AlCl3) assisted Friedel–Crafts reaction of dichloroaminoboranes, which are obtained from the reaction of aromatic amines with boron trichloride. Although long known,12 this concept has been reintroduced only recently.13 Similar to the Friedel–Crafts reaction, the photocyclisation of suitable BN stilbenes has almost been forgotten. Early investigations show that B,B,N-triarylaminoboranes form BN phenanthrenes upon irradiation.14 As photocyclisation of stilbenoid systems proves to be a versatile tool in all-carbon chemistry,15 the conceptual transfer to BN systems may also be a powerful tool for building up BN-PAHs in an atom-precise way. We reintroduce here the concept of photocyclisation for BN-PAHs and expand its scope by presenting two representatives of a new class of extended BN polyaromatic hydrocarbons that are related to perylenes. A photochemical synthesis is carried out to produce 1 whereas 2 is obtained upon hydrolysis of 1 (Fig. 1).
 | ||
| Fig. 1 Compound 1 is synthesized photochemically and fragments to 2 if treated with MeOH; 1 and 2 both bearing the perylene motif. | ||
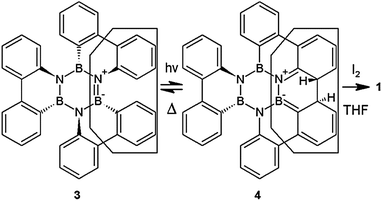
The synthesis of 1 is straightforward and can be accomplished by irradiation of 1,2:3,4:5,6-tris(o,o′-biphenylylene)borazine 316 in the presence of iodine–THF as an oxidant-scavenger system using a high pressure mercury vapour lamp in an overnight (15 h) reaction. Following the stilbene photocyclization reaction path the trans-dihydro intermediate 4 can also be assumed (Scheme 1). For best results, the reaction was carried out with 3 eq. of iodine (43% yield). Lowering the iodine load to 1 eq. resulted in a decrease to 27% yield, while raising it to 30 eq. mainly returned the starting material with only traces of 1. For safety and performance reasons, THF is preferred over propylene oxide as a scavenger of the formed hydrogen iodide prohibiting unwanted reaction interference.17 The reaction works well in toluene and cyclohexane, but owing to the low solubility of 3 in cyclohexane, suspensions of 3 have to be irradiated nearly 3 times longer than the solutions in toluene. Cyclohexene18 and DDQ19 have also been reported as oxidants in photocyclizations but they did not lead to product formation here. Higher concentrations (above 0.7 mM) were counterproductive and only led to a 20% yield of 1 with still some 25% of starting material 3 left unreacted.
The reaction mechanism is investigated by irradiating solutions of 1 in toluene with light of different wavelength ranges. Irradiation with light of 240–255 nm almost exclusively excites the solvent toluene and did not lead to product formation. Low conversion was obtained when the absorption bands of iodine were addressed (420–630 nm). Reasonable yields could only be achieved with light of 280–400 nm where toluene and iodine are almost transparent and 3 absorbs energy. This is in support of the proposed mechanism where 3 is directly excited to the S1 level and closes the ring upon relaxation in a conrotatory manner (6πa).20 This assumption was further confirmed by an unsuccessful reaction of 3 in cyclohexane with benzophenone as a triplet sensitizer that resulted in the recovery of the starting material.
Although stilbene photocyclisation is known for substrates with bromine substituents,21 the tribromo derivative 516c (Fig. 2) could not be cyclised as irradiation led to an almost complete decomposition of the starting material. Note that there are quite a number of all-carbon molecules known that, without a satisfying explanation, do not undergo stilbene photocyclisation.22
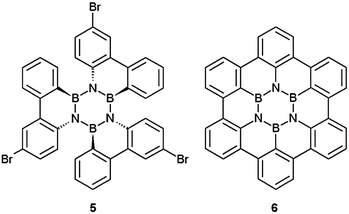 | ||
| Fig. 2 Tribromo derivative (5) of 3 and the ring closed planar hexa-peri-benzocoronene derivative 6. | ||
As the photocyclization worked reasonably well for closing one C–C-bond in our system, the possibility of forming three new C–C was investigated. But the synthesis of the BN-HBC derivative 6 (Fig. 2) was unsuccessful, even after irradiation of 1 for 62 hours and of 3 for 160 hours.
Compound 1 can be regarded as a BN phenanthrene fused to a B2N2 substituted dibenzoperylene. The geometrical constraint imposed by the dibenzoperylene unit is expected to impose on the borazine core. And indeed, treatment of 1 with methanol or wet CH2Cl2 yielded another BN-PAH 2 (Fig. 3).‡
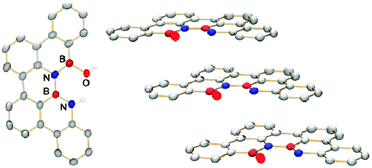
Crystals of 2 grew from a solution of 1 in a mixture of methanol and dichloromethane. They show positional disorder as the boron, nitrogen and oxygen sites are occupied in 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66 probability by these elements. Molecule 2 crystallizes in the γ motif (P212121) according to the shortest cell axis definition (2, (a): 5.2893(3) Å; 4.6 Å < γ < 5.4 Å) given by Desiraju and Gavezzotti.23 The essentially planar molecules form parallel displaced stacks with pronounced overlap and only small contribution of C–H interactions from neighbouring stacks. The distance between two molecules in a stack is 3.34 Å, while the displacement is 3.80 Å (long axis of 2) and 2.20 Å (short axis). Every stack is tilted toward the nearest stack in the c direction by 70° and to the nearest stack in the b direction by 78° (Fig. 3).
0.66 probability by these elements. Molecule 2 crystallizes in the γ motif (P212121) according to the shortest cell axis definition (2, (a): 5.2893(3) Å; 4.6 Å < γ < 5.4 Å) given by Desiraju and Gavezzotti.23 The essentially planar molecules form parallel displaced stacks with pronounced overlap and only small contribution of C–H interactions from neighbouring stacks. The distance between two molecules in a stack is 3.34 Å, while the displacement is 3.80 Å (long axis of 2) and 2.20 Å (short axis). Every stack is tilted toward the nearest stack in the c direction by 70° and to the nearest stack in the b direction by 78° (Fig. 3).
Consistent with the known BN substitution effect of increasing the HOMO–LUMO gap8b is the finding that the BN-perylenes 1 and 2 show hypsochromic shifts of around 70 nm for the longest absorbing bands compared to the parent 2:3,10:11-dibenzoperylene (λmax 440 nm).24 The fluorescence spectrum of 1 with its maxima at 371 and 389 nm is blue-shifted compared to 2 (386 and 399 nm) and shows a pronounced fine structure in contrast to the excitation spectrum as well as the UV-vis spectrum (Fig. 4). A direct comparison of the spectra of 1 and 2 with those of the corresponding all-carbon analogues is not possible as, to our knowledge, they are not known.
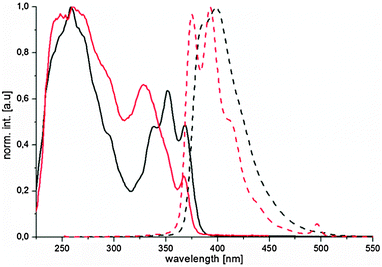 | ||
| Fig. 4 Fluorescence spectra in dichloromethane (red: 1; black: 2): excitation (solid lines, both 0 order) and emission (dotted lines, ex. 247 nm (red), 250 nm (black)). | ||
We have shown that the irradiation of 1:2;3:4;5:6-tris(o,o′-biphenylylene)borazine 3 yields the ring-closed molecule 1 that belongs to a BN substituted perylene series. Although the mechanism of its formation is still under investigation it seems reasonable to assume a stilbene-like photocyclisation reaction that proceeds by direct excitation of the precursor molecule 3. Upon hydrolysis of 1 another representative (2) of the aforementioned PAH class is formed.
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der chemischen Industrie through a fellowship to M.M.
Notes and references
- (a) L. Ci, L. Song, C. Jin, D. Jariwala, D. Wu, Y. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu and P. M. Ajayan, Nat. Mater., 2010, 9, 430 CrossRef CAS PubMed; (b) H. Liu, Y. Liu and D. Zhu, J. Mater. Chem., 2011, 21, 3335 RSC; (c) G. Imamura, C. W. Chang, Y. Nabae, M.-a. Kakimoto, S. Miyata and K. Saiki, J. Phys. Chem. C, 2012, 116, 16305 CrossRef CAS.
- M. J. D. Bosdet, C. A. Jaska, W. E. Piers, T. S. Sorensen and M. Parvez, Org. Lett., 2007, 9, 1395 CrossRef CAS PubMed.
- (a) L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, E. Moons, R. H. Friend and J. D. MacKenzie, Science, 2001, 293, 1119 CrossRef CAS PubMed; (b) J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718 CrossRef CAS PubMed; (c) D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS PubMed; (d) C. R. Dean, A. F. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim, K. L. Shepard and J. Hone, Nat. Nanotechnol., 2010, 5, 722 CrossRef CAS PubMed; (e) M. Bokdam, P. A. Khomyakov, G. Brocks, Z. Zhong and P. J. Kelly, Nano Lett., 2011, 11, 4631 CrossRef CAS PubMed; (f) K. Zhang, F. L. Yap, K. Li, C. T. Ng, L. J. Li and K. P. Loh, Adv. Funct. Mater., 2014, 731 CrossRef CAS.
- (a) J. da Rocha Martins and H. Chacham, ACS Nano, 2011, 5, 385 CrossRef PubMed; (b) C.-K. Chang, S. Kataria, C.-C. Kuo, A. Ganguly, B.-Y. Wang, J.-Y. Hwang, K.-J. Huang, W.-H. Yang, S.-B. Wang, C.-H. Chuang, M. Chen, C.-I. Huang, W.-F. Pong, K.-J. Song, S.-J. Chang, J.-H. Guo, Y. Tai, M. Tsujimoto, S. Isoda, C.-W. Chen, L.-C. Chen and K.-H. Chen, ACS Nano, 2013, 7, 1333 CrossRef CAS PubMed.
- G. Giovannetti, P. A. Khomyakov, G. Brocks, P. J. Kelly and J. van den Brink, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 073103 CrossRef.
- M. J. D. Bosdet, W. E. Piers, T. S. Sorensen and M. Parvez, Angew. Chem., Int. Ed., 2007, 46, 4940 CrossRef CAS PubMed.
- (a) M. J. D. Bosdet and W. E. Piers, Can. J. Chem., 2009, 87, 8 CrossRef; (b) P. G. Campbell, A. J. V. Marwitz and S.-Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6074 CrossRef CAS PubMed.
- (a) G. C. Culling, M. J. S. Dewar and P. A. Marr, J. Am. Chem. Soc., 1964, 86, 1125 CrossRef CAS; (b) C. Tönshoff, M. Müller, T. Kar, F. Latteyer, T. Chassé, K. Eichele and H. F. Bettinger, ChemPhysChem, 2012, 13, 1173 CrossRef PubMed; (c) S. Biswas, M. Müller, C. Tönshoff, K. Eichele, C. Maichle-Mössmer, A. Ruff, B. Speiser and H. F. Bettinger, Eur. J. Org. Chem., 2012, 4634 CrossRef CAS.
- (a) M. Dewar and R. Jones, J. Am. Chem. Soc., 1968, 90, 2137 CrossRef CAS; (b) M. J. S. Dewar and R. Dietz, J. Chem. Soc., 1959, 2728 RSC; (c) D. G. White, J. Am. Chem. Soc., 1963, 85, 3634 CrossRef CAS; (d) T. Riehm, H. Wadepohl and L. H. Gade, Inorg. Chem., 2008, 47, 11467 CrossRef CAS PubMed.
- (a) C. A. Jaska, D. J. H. Emslie, M. J. D. Bosdet, W. E. Piers, T. S. Sorensen and M. Parvez, J. Am. Chem. Soc., 2006, 128, 10885 CrossRef CAS PubMed; (b) C. A. Jaska, W. E. Piers, R. McDonald and M. Parvez, J. Org. Chem., 2007, 72, 5234 CrossRef CAS PubMed; (c) M. J. D. Bosdet, W. E. Piers, T. S. Sorensen and M. Parvez, Can. J. Chem., 2010, 88, 426 CrossRef CAS PubMed; (d) J. J. Wadle, L. B. McDermott and E. H. Fort, Tetrahedron Lett., 2014, 55, 445 CrossRef CAS PubMed.
- (a) A. J. Ashe and X. Fang, Org. Lett., 2000, 2, 2089 CrossRef CAS PubMed; (b) A. J. Ashe, X. Fang, X. Fang and J. W. Kampf, Organometallics, 2001, 20, 5413 CrossRef CAS; (c) A. J. Ashe, H. Yang, X. Fang and J. W. Kampf, Organometallics, 2002, 21, 4578 CrossRef CAS; (d) X. Fang, H. Yang, J. W. Kampf, M. M. Banaszak Holl and A. J. Ashe, Organometallics, 2006, 25, 513 CrossRef CAS; (e) A. J. V. Marwitz, M. H. Matus, L. N. Zakharov, D. A. Dixon and S.-Y. Liu, Angew. Chem., Int. Ed., 2009, 48, 973 CrossRef CAS PubMed; (f) J. Pan, J. Wang, M. M. Banaszak Holl, J. W. Kampf and A. J. Ashe, Organometallics, 2006, 25, 3463 CrossRef CAS.
- (a) M. J. S. Dewar, V. P. Kubba and R. Pettit, J. Chem. Soc., 1958, 3073 RSC; (b) M. J. S. Dewar and W. H. Poesche, J. Org. Chem., 1964, 29, 1757 CrossRef CAS.
- (a) T. Hatakeyama, S. Hashimoto, T. Oba and M. Nakamura, J. Am. Chem. Soc., 2012, 134, 19600 CrossRef CAS PubMed; (b) T. Hatakeyama, S. Hashimoto, S. Seki and M. Nakamura, J. Am. Chem. Soc., 2011, 133, 18614 CrossRef CAS PubMed; (c) X.-Y. Wang, H.-R. Lin, T. Lei, D.-C. Yang, F.-D. Zhuang, J.-Y. Wang, S.-C. Yuan and J. Pei, Angew. Chem., Int. Ed., 2013, 52, 3117 CrossRef CAS PubMed; (d) S. R. Wisniewski, C. L. Guenther, O. A. Argintaru and G. A. Molander, J. Org. Chem., 2014, 79, 365 CrossRef CAS PubMed.
- (a) M. E. Glogowski, P. J. Grisdale, J. L. R. Williams and T. H. Regan, J. Organomet. Chem., 1973, 54, 51 CrossRef; (b) M. E. Glogowski and J. L. R. Williams, J. Organomet. Chem., 1980, 195, 123 CrossRef CAS; (c) P. J. Grisdale, M. E. Glogowski and J. L. R. Williams, J. Org. Chem., 1971, 36, 3821 CrossRef CAS; (d) P. J. Grisdale and J. L. R. Williams, J. Org. Chem., 1969, 34, 1675 CrossRef CAS; (e) K. G. Hancock and D. A. Dickinson, J. Am. Chem. Soc., 1973, 95, 280 CrossRef CAS; (f) K. D. Müller and K. Niedenzu, Inorg. Chim. Acta, 1977, 25, L53 CrossRef; (g) R. F. Porter and L. J. Turbini, Photochemistry of boron compounds, Springer, Berlin, Heidelberg, 1981 Search PubMed.
- (a) R. H. Martin, Angew. Chem., Int. Ed. Engl., 1974, 13, 649 CrossRef; (b) H. Meier, Angew. Chem., Int. Ed. Engl., 1992, 31, 1399 CrossRef; (c) K. B. Jørgensen, Molecules, 2010, 15, 4334 CrossRef PubMed; (d) M. Gingras, Chem. Soc. Rev., 2013, 42, 968 RSC.
- (a) R. Köster, H. Bellut and S. Hattori, Liebigs Ann. Chem., 1968, 720, 1 CrossRef; (b) R. Köster, K. Iwasaki, S. Hattori and Y. Morita, Liebigs Ann. Chem., 1968, 720, 23 CrossRef; (c) M. Müller, C. Maichle-Mössmer, P. Sirsch and H. F. Bettinger, ChemPlusChem, 2013, 78, 988 CrossRef.
- L. Liu, B. Yang, T. J. Katz and M. K. Poindexter, J. Org. Chem., 1991, 56, 3769 CrossRef CAS.
- H. R. Talele, M. J. Gohil and A. V. Bedekar, Bull. Chem. Soc. Jpn., 2009, 82, 1182 CrossRef CAS.
- (a) F. B. Mallory, C. S. Wood and J. T. Gordon, J. Am. Chem. Soc., 1964, 86, 3094 CrossRef CAS; (b) A. C. Hernandez-Perez, A. Vlassova and S. K. Collins, Org. Lett., 2012, 14, 2988 CrossRef CAS PubMed.
- (a) T. J. H. M. Cuppen and W. H. Laarhoven, J. Am. Chem. Soc., 1972, 94, 5914 CrossRef CAS; (b) C.-W. Jiang, R.-H. Xie, F.-L. Li and R. E. Allen, Chem. Phys. Lett., 2010, 487, 177 CrossRef CAS PubMed; (c) I. N. Ioffe and A. A. Granovsky, J. Chem. Theory Comput., 2013, 9, 4973 CrossRef CAS; (d) N. Ito, T. Hirose and K. Matsuda, Org. Lett., 2014, 16, 2502 CrossRef CAS PubMed; (e) M. S. Molloy, J. A. Snyder and A. E. Bragg, J. Phys. Chem. A, 2014 DOI:10.1021/jp501988g.
- (a) A. Sudhakar and T. J. Katz, J. Am. Chem. Soc., 1986, 108, 179 CrossRef CAS; (b) L. Liu and T. J. Katz, Tetrahedron Lett., 1991, 32, 6831 CrossRef CAS; (c) F. B. Mallory, K. E. Butler, A. Bérubé, E. D. Luzik Jr, C. W. Mallory, E. J. Brondyke, R. Hiremath, P. Ngo and P. J. Carroll, Tetrahedron, 2001, 57, 3715 CrossRef CAS.
- E. V. Blackburn and C. J. Timmons, J. Chem. Soc. C, 1970, 172 RSC.
- (a) G. R. Desiraju and A. Gavezzotti, Acta Crystallogr., Sect. B: Struct. Sci., 1989, 45, 473 CrossRef; (b) G. R. Desiraju and A. Gavezzotti, J. Chem. Soc., Chem. Commun., 1989, 621 RSC.
- E. Clar and M. Zander, J. Chem. Soc., 1958, 1861 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and spectra of 1 and 2, and the Raman spectrum of 3. CCDC 987402. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc01424c |
| ‡ Reaction of 1 with methanol also produces 2, presumably due to instability of the expected methoxy derivative to H2O. |
| This journal is © The Royal Society of Chemistry 2014 |