Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electron-donor function of methanofullerenes in donor–acceptor bulk heterojunction systems†
Yutaka
Ie
*ab,
Makoto
Karakawa
a,
Seihou
Jinnai
a,
Hiroyuki
Yoshida
bc,
Akinori
Saeki
bd,
Shu
Seki
d,
Shunsuke
Yamamoto
e,
Hideo
Ohkita
be and
Yoshio
Aso
*a
aThe Institute of Scientific and Industrial Research (ISIR), Osaka University, 8-1 Mihogaoka, Ibaraki, Osaka 567-0047, Japan. E-mail: yutakaie@sanken.osaka-u.ac.jp; aso@sanken.osaka-u.ac.jp
bJapan Science and Technology Agency (JST)-PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama 333-0012, Japan
cInstitute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan
dDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
eDepartment of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo, Kyoto 615-8510, Japan
First published on 26th February 2014
Abstract
Electron-donor function of methanofullerenes (MFs) in bulk heterojunction systems is demonstrated by the combination of MFs with the electron-transporting π-system that has a much higher electron affinity than MFs.
Fullerene derivatives have been well-recognized as electron-accepting materials and extensively used in donor–acceptor linked systems in order to investigate a photoinduced charge-separation process in the molecular level.1 This fundamental aspect is extended to the photon-to-current conversion process in bulk composite devices. In fact, organic photovoltaics (OPVs) have become an active area of research in both academia and industry in recent years.2 The most efficient organic layers for OPVs are based on the concept of bulk heterojunctions (BHJs), which are composed of a blend of electron-donor (p-type) and electron-acceptor (n-type) materials.3 Methanofullerene derivatives represented by [6,6]-phenyl-Cx-butyric acid methyl ester (x = 61: PC61BM, x = 71: PC71BM) have been employed as typical acceptor semiconductors in such systems (Fig. 1).4 On the other hand, the hole-transporting characteristics of the methanofullerenes in bulk heterojunction films remain unclear, although there have been several reports suggesting that fullerene derivatives participate in not only electron transportation but also hole transportation in donor polymer–PC61BM blend films.5,6 Considering that PC61BM shows ambipolar charge-transport characteristics,7 it should also be able to function as a donor semiconductor when combined with acceptor materials whose low-lying lowest unoccupied molecular orbital (LUMO) energy levels are much lower than that of PC61BM. Nevertheless, the use of fullerene derivatives as donor materials is limited to the combination of fullerene (C60)/perfluorinated phthalocyanine derivatives in vacuum-deposited bilayer OPV devices.8,9 In addition, the confined interfaces between the donor and acceptor materials in such bilayer systems hinder spectroscopic investigations.
In order to realize BHJ systems that employ PC61BM as the donor material, it is necessary to use acceptor materials with low LUMO energy levels, high electron mobilities, and good solubilities. Recently, we developed a new electron-transporting π-conjugated compound (BCN-HH-BCN) that meets these criteria (Fig. 1).10 We envisioned that using BCN-HH-BCN in combination with PC61BM should allow the electron-donor function and hole-transporting characteristics of PC61BM in blend films to be elucidated. In this communication, we systematically investigated the material properties, the photovoltaic characteristics of BHJ solar cells based on PC61BM and BCN-HH-BCN, and the charge carrier dynamics in the corresponding blend films.
Previously, the electrochemical properties of BCN-HH-BCN were investigated by cyclic voltammetry measurements performed in a fluorobenzene solution.10 On the basis of its half-wave reduction potential (−0.67 V vs. Fc/Fc+) and considering that the energy level of ferrocene/ferrocenium (Fc/Fc+) is −4.8 eV below the vacuum level, the LUMO energy level of BCN-HH-BCN was estimated to be −4.1 eV. Although this method is widely used for estimating HOMO and LUMO energies, the values determined by this method are for isolated single molecules in solution only. In addition, these values depend on a number of factors such as different approximations of the formal potential of Fc/Fc+.11 Recently, low-energy inverse photoemission spectroscopy (LEIPS) has been demonstrated. It involves the use of irradiating electrons with kinetic energies lower than the damage threshold of organic materials.12a The photon energy falls in the near-ultraviolet range, allowing one to use a multilayer bandpass filter for photon detection. This leads to an overall energy resolution of 0.3 eV or better. In this study, we applied this new technique to determine the LUMO energy level of BCN-HH-BCN in the solid state. Fig. 2(a) shows the LEIP spectra of BCN-HH-BCN and PC61BM obtained at a photon energy of 5.0 eV. On the basis of the onset energy with respect to the vacuum level, the electron affinity of BCN-HH-BCN in the solid state is determined to be −4.45 eV (see also Fig. S1 in the ESI†). Since the electron affinity of PC61BM was recently determined to be −3.81 eV by the same technique,12b as shown in Fig. 2(a), the LUMO energy level of BCN-HH-BCN is 0.64 eV lower than that of PC61BM.
 | ||
| Fig. 2 (a) LEIP spectra of BCN-HH-BCN (blue) and PC61BM (red).12b (b) Energy level diagrams for BCN-HH-BCN and PC61BM. The HOMO and LUMO energy levels were determined by the PESA and LEIPS, respectively. | ||
The ionization potentials (IPs) of BCN-HH-BCN and PC61BM films were measured by photoelectron spectroscopy in air (PESA). The IP for BCN-HH-BCN was determined to be −6.64 eV, which is 0.56 eV lower than that of PC61BM (Fig. S2, ESI†). On the basis of these results, the energy levels of BCN-HH-BCN and PC61BM were unambiguously determined and are depicted in Fig. 2(b); it can be inferred that BCN-HH-BCN is a good candidate for an acceptor material to cause the donor function of PC61BM.
The local hole mobility of PC61BM was investigated by flash-photolysis time-resolved microwave conductivity (FP-TRMC).13 As shown in Fig. S3(a) (ESI†), the photoconductivity transient of a PC61BM film prepared by the drop casting of a PC61BM solution in tetrahydrofuran (THF) and excited at 355 nm displays a maximum ϕΣμ of 2.6 × 10−5 cm2 V−1 s−1, where ϕ and Σμ denote the charge carrier generation yield and the sum of the hole and electron mobilities (Σμ = μ+ + μ−), respectively. Upon the addition of tetracyanoethylene (TCNE) in the blend ratio of PC61BM/TCNE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 by weight, the maximum ϕΣμ was increased to 3.2 × 10−5 cm2 V−1 s−1. This is due to electron transfer from PC61BM to TCNE, which acts as a strong electron acceptor against not only donor polymers (e.g., poly(3-hexylthiophene))14a but also PC61BM.6a Concomitantly, the photocurrent, measured using a comb-type interdigitated Au electrode on glass, increased by 65%, as shown in Fig. S3(b) (ESI†). Because the amount of TCNE added is low (10%) and since TCNE lacks an effective electron transporting pathway, the increases in both the FP-TRMC and the photocurrent transients are most likely because of the photogenerated holes in PC61BM. By estimating ϕ using a previously reported procedure,14b the local hole mobility of PC61BM is found to be 8 × 10−3 cm2 V−1 s−1. Meanwhile, the electron mobility in pristine PC61BM, obtained in the same fashion, was 0.02 cm2 V−1 s−1 and in agreement with that reported previously (0.04–0.3 cm2 V−1 s−1) using pulse radiolysis TRMC.7b The fact that the hole mobility of PC61BM is lower than its electron mobility as well as its deep LUMO is indicative of the primary n-type nature of PC61BM; this property is the reason it is commonly used in OPVs and field-effect transistors. However, PC61BM is potentially able to serve as a donor in combination with a much stronger electron acceptor.
0.1 by weight, the maximum ϕΣμ was increased to 3.2 × 10−5 cm2 V−1 s−1. This is due to electron transfer from PC61BM to TCNE, which acts as a strong electron acceptor against not only donor polymers (e.g., poly(3-hexylthiophene))14a but also PC61BM.6a Concomitantly, the photocurrent, measured using a comb-type interdigitated Au electrode on glass, increased by 65%, as shown in Fig. S3(b) (ESI†). Because the amount of TCNE added is low (10%) and since TCNE lacks an effective electron transporting pathway, the increases in both the FP-TRMC and the photocurrent transients are most likely because of the photogenerated holes in PC61BM. By estimating ϕ using a previously reported procedure,14b the local hole mobility of PC61BM is found to be 8 × 10−3 cm2 V−1 s−1. Meanwhile, the electron mobility in pristine PC61BM, obtained in the same fashion, was 0.02 cm2 V−1 s−1 and in agreement with that reported previously (0.04–0.3 cm2 V−1 s−1) using pulse radiolysis TRMC.7b The fact that the hole mobility of PC61BM is lower than its electron mobility as well as its deep LUMO is indicative of the primary n-type nature of PC61BM; this property is the reason it is commonly used in OPVs and field-effect transistors. However, PC61BM is potentially able to serve as a donor in combination with a much stronger electron acceptor.
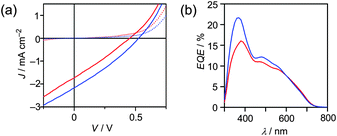
In order to investigate the photovoltaic properties of a blend of PC61BM and BCN-HH-BCN, a conventional BHJ solar cell was fabricated. The current density–voltage (J–V) characteristics of the device were evaluated under air mass 1.5 global (AM1.5G) simulated solar illumination with an irradiation intensity of 100 mW cm−2. The configuration of the cell was as follows: glass/indium tin oxide (ITO)/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)(PEDOT:PSS)/active layer/Al. The conditions for fabricating the active layer were optimized and found that an active layer could be prepared by spin coating an o-dichlorobenzene solution of the PC61BM/BCN-HH-BCN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) blend in a nitrogen atmosphere without thermal annealing (see the ESI†). Under these optimized conditions, the OPV device exhibited a short-circuit current (JSC) of 1.73 mA cm−2, an open-circuit voltage (VOC) of 0.45 V, a fill factor (FF) of 0.27, and a PCE of 0.21% (Fig. 3(a)). The photocurrent action spectrum of the external quantum efficiency (EQE) of the device was measured to reveal its photoresponse against different wavelengths. As shown in Fig. 3(b), the EQE spectrum of the device exhibited a broad response in the range of 300–750 nm. This profile resembled well the absorption spectrum of a PC61BM/BCN-HH-BCN (2
1 weight ratio) blend in a nitrogen atmosphere without thermal annealing (see the ESI†). Under these optimized conditions, the OPV device exhibited a short-circuit current (JSC) of 1.73 mA cm−2, an open-circuit voltage (VOC) of 0.45 V, a fill factor (FF) of 0.27, and a PCE of 0.21% (Fig. 3(a)). The photocurrent action spectrum of the external quantum efficiency (EQE) of the device was measured to reveal its photoresponse against different wavelengths. As shown in Fig. 3(b), the EQE spectrum of the device exhibited a broad response in the range of 300–750 nm. This profile resembled well the absorption spectrum of a PC61BM/BCN-HH-BCN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend film (Fig. S4(a), ESI†). On the basis of the absorption spectra of PC61BM and BCN-HH-BCN in the solid state (Fig. S4(b), ESI†), it can be said that the photoresponses of the device in the short-wavelength region of approximately 400 nm and the long-wavelength region extending from 500 to 750 nm are mainly derived from PC61BM and BCN-HH-BCN, respectively. This indicates that, on being photoexcited, both PC61BM and BCN-HH-BCN contributed to the photocurrent generated. Furthermore, as shown in Fig. 3(a), the PCE was improved to 0.34% for the OPV device based on PC71BM and BCN-HH-BCN (2
1) blend film (Fig. S4(a), ESI†). On the basis of the absorption spectra of PC61BM and BCN-HH-BCN in the solid state (Fig. S4(b), ESI†), it can be said that the photoresponses of the device in the short-wavelength region of approximately 400 nm and the long-wavelength region extending from 500 to 750 nm are mainly derived from PC61BM and BCN-HH-BCN, respectively. This indicates that, on being photoexcited, both PC61BM and BCN-HH-BCN contributed to the photocurrent generated. Furthermore, as shown in Fig. 3(a), the PCE was improved to 0.34% for the OPV device based on PC71BM and BCN-HH-BCN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): a JSC of 2.17 mA cm−2, a VOC of 0.53 V, and a FF of 0.29. The EQE spectrum of the PC71BM-containing device showed a higher efficiency in the visible region than that of the PC61BM-containing device (Fig. 3(a)), reflecting the relatively high absorbance of PC71BM (Fig. S4(a), ESI†). In addition, hole-only devices with a structure of ITO/PEDOT:PSS/PC61BM or PC71BM/Au showed that the space-charge-limited current (SCLC) hole mobility of PC71BM (1.6 × 10−6 cm2 V−1 s−1) was slightly higher than that of PC61BM (1.4 × 10−6 cm2 V−1 s−1) (Fig. S5, ESI†). This result also supports the improved performance of the PC71BM-containing device.
1): a JSC of 2.17 mA cm−2, a VOC of 0.53 V, and a FF of 0.29. The EQE spectrum of the PC71BM-containing device showed a higher efficiency in the visible region than that of the PC61BM-containing device (Fig. 3(a)), reflecting the relatively high absorbance of PC71BM (Fig. S4(a), ESI†). In addition, hole-only devices with a structure of ITO/PEDOT:PSS/PC61BM or PC71BM/Au showed that the space-charge-limited current (SCLC) hole mobility of PC71BM (1.6 × 10−6 cm2 V−1 s−1) was slightly higher than that of PC61BM (1.4 × 10−6 cm2 V−1 s−1) (Fig. S5, ESI†). This result also supports the improved performance of the PC71BM-containing device.
We measured the transient absorption spectra (TAS) of the PC61BM–BCN-HH-BCN blend film to elucidate its charge generation dynamics. As shown in Fig. 4(a), 0.1 ns after the laser excitation, a sharp absorption peak was observed at approximately 700 nm. In addition, a broad absorption band was observed extending from 900 to 1200 nm. The absorption band at 700–900 nm disappeared gradually and instead an absorption band was observed at approximately 1100 nm at 1 ns after the excitation. In order to assign these absorption bands, we measured the absorption spectra of the BCN-HH-BCN singlet excitons, triplet excitons, and radical anions separately (see the ESI†). Consequently, we found that the BCN-HH-BCN radical anions, singlet excitons, and triplet excitons exhibit absorption bands at 720, 950, and 1100 nm, respectively. As reported previously, PC61BM radical cations exhibit an absorption band at 890 nm.6a On the basis of this fact, the transient spectrum at 0.1 ns could be replicated by summing the individual spectrum of the PC61BM radical cations, the BCN-HH-BCN radical anions, and the BCN-HH-BCN triplet excitons, as shown in Fig. 4(b). This finding clearly shows that PC61BM radical cations and BCN-HH-BCN radical anions are generated in the blend upon photoexcitation. On the other hand, the transient spectrum at 1 ns was almost identical to the absorption spectrum of the BCN-HH-BCN triplet excitons. This triplet formation cannot be ascribed to the intersystem crossing because BCN-HH-BCN singlet excitons disappeared in 2 ps in PC61BM–BCN-HH-BCN films. Rather, it is ascribed to the triplet formation through the charge recombination of PC61BM radical cations and BCN-HH-BCN radical anions. This is probably because BCN-HH-BCN triplet excitons are more stable than both PC61BM radical cations and BCN-HH-BCN radical anions. The triplet energy (ET) of BCN-HH-BCN is roughly estimated to be 1.1 eV, assuming that the difference in the energies of singlet and triplet excitons is 0.7 eV, which is the typical value for small molecules.15 On the other hand, the triplet energy of PC61BM is reported to be 1.5 eV.16 The energy of the charge-separated states (ECT) was estimated to be 1.63 eV from the HOMO (PC61BM) and LUMO (BCN-HH-BCN) levels shown in Fig. 2(b). We, therefore, conclude that PC61BM radical cations and BCN-HH-BCN radical anions are generated in the blend but are converted to the more stable BCN-HH-BCN triplet states by charge recombination. This formation of the triplet states is consistent with the relatively low device performance of the PC61BM–BCN-HH-BCN solar cells.
In summary, we unambiguously determined the molecular properties of PC61BM and BCN-HH-BCN in the solid state. The results obtained suggest that PC61BM should function as a donor semiconductor when paired with a strong acceptor BCN-HH-BCN. As a consequence, BHJ solar cells based on a combination of PCxBM (x = 61 or 71) and BCN-HH-BCN showed photovoltaic characteristics. The transient absorption measurements of the blend film suggested the formation of PC61BM radical cations and BCN-HH-BCN radical anions. The most notable point in this study is that methanofullerene derivatives can contribute to hole transportation as p-type materials in BHJ systems, which provides important insights into the precise molecular design of semiconducting materials and/or new donor–acceptor systems.
This work was supported by the funding program, JST, JSPS, and MEXT, Japan. We thank Prof. Y. Murata and Dr A. Wakamiya of Kyoto University for PESA measurements.
Notes and references
- H. Imahori and S. Fukuzumi, Adv. Funct. Mater., 2004, 14, 525 CrossRef CAS; D. M. Guldi, B. M. Illescas, C. M. Atienza, M. Wielopolski and N. Martin, Chem. Soc. Rev., 2009, 38, 1587 RSC; F. D'Souza and O. Ito, Chem. Commun., 2009, 4913 RSC.
- Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed; A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Prog. Photovoltaics, 2013, 21, 1 Search PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 Search PubMed; M. Hiramoto, H. Fujiwara and M. Yokoyama, Appl. Phys. Lett., 1991, 58, 1062 CrossRef CAS PubMed.
- M. M. Wienk, J. M. Kroon, W. J. H. Verhees, J. Knol, J. C. Hummelen, P. A. van Hal and R. A. J. Janssen, Angew. Chem., Int. Ed., 2003, 42, 3371 CrossRef CAS PubMed; Y. He and Y. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970 RSC; Y. Matsuo, Chem. Lett., 2012, 41, 754 CrossRef.
- C. Melzer, E. J. Koop, V. D. Mihailetchi and P. W. M. Blom, Adv. Funct. Mater., 2004, 14, 865 CrossRef CAS; S. M. Tuladhar, D. Poplavskyy, S. A. Choulis, J. R. Durrant, D. D. C. Bradley and J. Nelson, Adv. Funct. Mater., 2005, 15, 1171 CrossRef; A. Gadisa, K. Tvingstedt, K. Vandewal, F. Zhang, J. V. Manca and O. Inganäs, Adv. Mater., 2010, 22, 1008 CrossRef PubMed.
- (a) S. Yamamoto, J. Guo, H. Ohkita and S. Ito, Adv. Funct. Mater., 2008, 15, 2555 CrossRef; (b) S. Yamamoto, H. Ohkita, H. Benten and S. Ito, Adv. Funct. Mater., 2012, 22, 3075 CrossRef CAS.
- (a) T. D. Anthopoulos, C. Tanase, S. Setayesh, E. J. Meijer, J. C. Hummelen, P. W. M. Blom and D. M. de Leeuw, Adv. Mater., 2004, 16, 2174 CrossRef CAS; (b) M. P. de Haas, J. M. Warman, T. D. Anthopoulos and D. M. de Leeuw, Adv. Funct. Mater., 2006, 16, 2274 CrossRef CAS.
- Q. L. Song, H. B. Yang, Y. Gan, C. Gong and C. M. Li, J. Am. Chem. Soc., 2010, 132, 4554 CrossRef CAS PubMed; J. L. Yang, P. Sullivan, S. Schumann, I. Hancox and T. S. Jones, Appl. Phys. Lett., 2012, 100, 023307 CrossRef PubMed.
- Metal-doped C60 can also function as a donor: M. Kubo, K. Iketaki, T. Kaji and M. Hiramoto, Appl. Phys. Lett., 2011, 98, 073311 CrossRef PubMed.
- Y. Ie, K. Nishida, M. Karakawa, H. Tada, A. Asano, A. Saeki, S. Seki and Y. Aso, Chem.–Eur. J., 2011, 17, 4750 CrossRef CAS PubMed.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367 CrossRef CAS PubMed.
- (a) H. Yoshida, Chem. Phys. Lett., 2012, 539–540, 180 CrossRef CAS PubMed; (b) H. Yoshida, MRS Proc., 2012, 1493, 295 Search PubMed.
- A. Saeki, Y. Koizumi, T. Aida and S. Seki, Acc. Chem. Res., 2012, 45, 1193 CrossRef CAS PubMed.
- (a) A. Saeki, S. Seki, Y. Koizumi, T. Sunagawa, K. Ushida and S. Tagawa, J. Phys. Chem. B, 2005, 109, 10015 CrossRef CAS PubMed; (b) Y. Yasutani, A. Saeki, T. Fukumatsu, Y. Koizumi and S. Seki, Chem. Lett., 2013, 42, 19 CrossRef CAS.
- A. Köhler and H. Bässler, Mater. Sci. Eng., R, 2009, 66, 71 CrossRef PubMed.
- S. Cook, H. Ohkita, J. R. Durrant, Y. Kim, J. J. Benson-Smith, J. Nelson and D. D. C. Bradley, Appl. Phys. Lett., 2006, 89, 101128 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: LEIP, PESA, TRMC, SCLC, and UV-vis measurements; detailed measurement conditions. See DOI: 10.1039/c4cc00940a |
| This journal is © The Royal Society of Chemistry 2014 |