Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Axially chiral BODIPYs†
Reinner I.
Lerrick
ab,
Thomas P. L.
Winstanley
a,
Karen
Haggerty
a,
Corinne
Wills
a,
William
Clegg
a,
Ross W.
Harrington
a,
Patrick
Bultinck
c,
Wouter
Herrebout
d,
Andrew C.
Benniston
a and
Michael J.
Hall
*a
aSchool of Chemistry, Bedson Building, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: m.hall@ncl.ac.uk; Fax: +44 (0)191 222 6929; Tel: +44 (0)191 222 7321
bSchool of Chemistry, Nusa Cendana University, Indonesia
cDepartment of Inorganic and Physical Chemistry, Ghent University, Krijgslaan 281-S3, 9000 Gent, Belgium
dDepartment of Chemistry, University of Antwerp, Groenenborgerlaan 171, 2020 Antwerp, Belgium
First published on 19th March 2014
Abstract
The synthesis and resolution of a class of chiral organic fluorophores, axially chiral 4,4-difluoro-4-bora-3a,4a-diaza-s-indacenes (Ax*-BODIPY), is described. Ax*-BODIPYs were prepared through a modular synthesis combined with a late stage Heck functionalisation. Resolution was achieved by preparative chiral HPLC. Absolute stereochemical assignment was performed by comparison of experimental ECD spectra with TD-DFT calculations.
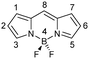
The boron-dipyrromethene dyes (BODIPY)1 are among the most widely used organic fluorophores, finding utility in an array of applications including photodynamic therapy,2 biological imaging3 and fluorescence sensing.4 The continuing popularity of the BODIPYs derives from the combination of robust synthetic protocols with desirable physical properties such as thermal, chemical and photochemical stability, high fluorescence quantum yield, low intrinsic triplet-state formation, high extinction coefficients and good solubility (Fig. 1).5
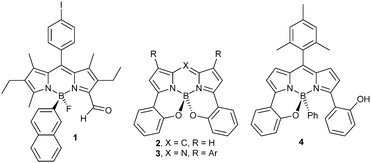
Chiral fluorophores have been explored for the selective sensing of chiral molecules,6 including attempts to determine the enantiomeric excess of a solution by optical measurements.7 A number of chiral fluorophores have been reported, the most widely studied being the lanthanide coordination complexes,8 1,1′-bi-2-naphthols (BINOL) and helicenes.9 Chiral molecules containing the BODIPY fluorophore have been synthesised, typically through the decoration of the BODIPY core with chiral appendages.10 An example of a resolved BODIPY based around an asymmetric boron atom (B*-BODIPY) with the chirality embedded in the core structure of the fluorophore itself has been described (1),11 whilst a number of unresolved boron-centred chiral BODIPYs have been reported based on the intramolecularly B–O bonded BODIPY and the closely related aza-BODIPY systems (2–4) (Fig. 2).12
In this manuscript we present a general approach for the synthesis, resolution and absolute stereochemical determination of a class of axially chiral BODIPYs (Ax*-BODIPY), based on restricted rotation of aromatic substituents in the meso-position (or 8-position).
The rotation of aryl substituents at the meso-position of BODIPYs has been previously studied because of the influence this has on both the S1 lifetime and the fluorescence quantum yield of the fluorophore.13 These results have been applied in the development of BODIPY “rotors” for fluorescence sensing of microenvironment viscosity.14
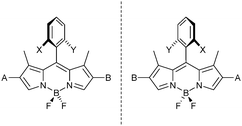
Our design strategy for a resolvable atropisomeric Ax*-BODIPY system therefore involved provision for (a) a high rotational barrier for an aryl substituent at the meso-position (b) chemically differentiable groups on the ortho-positions of the meso-aryl substituent (X and Y) and (c) chemically differentiable groups on the 2/6-positions (A and B) of the BODIPY core (Fig. 3).15
In order to ensure a high rotational barrier for ortho-substituted meso-aryl groups we opted to include methyl groups in both the 1- and 7-positions of the BODIPY fluorophore. The introduction of ortho-functionalised meso-aryl substituents was anticipated to be straightforward, through the application of condensation-based synthetic routes towards BODIPY systems. Finally to allow synthetic flexibility we decided to chemically differentiate at the 2/6-positions via a late stage metal-catalysed cross-coupling reaction.
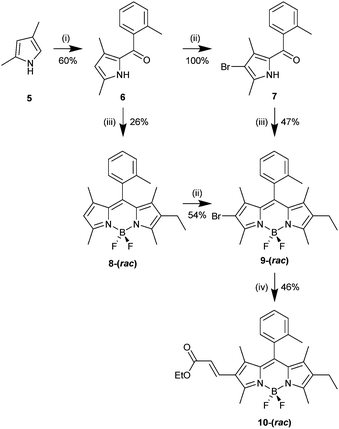
Synthesis of 10-(rac) started with the deprotonation of 2,4-dimethyl-1H-pyrrole (5) with EtMgBr and subsequent C-2 selective acylation with 2-methylbenzoyl chloride to give (3,5-dimethyl-1H-pyrrol-2-yl)(o-tolyl)methanone (6). Pyrrole 6 was then brominated at the 4-position to give (4-bromo-3,5-dimethyl-1H-pyrrol-2-yl)(o-tolyl)methanone (7), followed by acid-catalysed condensation with 3-ethyl-2,4-dimethyl-1H-pyrrole and subsequent BF2 chelation to give 9-(rac). A complementary route to 9-(rac) was also investigated involving condensation of pyrrole 6 with 3-ethyl-2,4-dimethyl-1H-pyrrole, BF2 chelation to give 8-(rac) and subsequent bromination. 9-(rac) is a useful starting point for the synthesis of different axially chiral BODIPY systems through the use of metal-catalysed cross-coupling reactions. In our case we chose to functionalise 9-(rac)via a Heck reaction with ethyl acrylate, differentiating the 2- and 6-positions, to give the target 10-(rac) (Scheme 1).
Compounds 8-(rac), 9-(rac) and 10-(rac) gave UV/Vis absorbance (λmax = 515, 526, 538 nm) and fluorescence (λmax = 525, 540, 555 nm) spectra typical of the BODIPY class of fluorophores, with high extinction coefficients and moderate to good fluorescence quantum yields (ESI†).
The asymmetry of compounds 8-(rac), 9-(rac) and 10-(rac) was observable by NMR spectroscopy, the 1H NMR spectrum showing five different methyl environments in each case. Restricted rotation of the meso-aryl group imposes a diastereotopic relationship on the two fluorine atoms. This was observable in the 19F NMR spectrum as an ABX coupling pattern, each fluorine peak showing both geminal 19F–19F and 19F–11B coupling (ESI† and Fig. 4).16
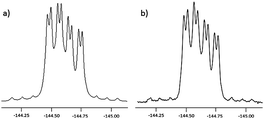 | ||
| Fig. 4 (a) Simulated (using gNMR)17 and (b) experimental 19F NMR spectra of 10-(rac). Note the asymmetry in the spectra due to 10B coupling. | ||
Crystallisation of both 9-(rac) and 10-(rac) by slow diffusion (DCM/petrol) gave single crystals suitable for X-ray analysis. 9-(rac) and 10-(rac) gave monoclinic crystals, both with the centrosymmetric space group P21/c. In both cases four molecules are present per unit cell, two of each of the (R)- and (S)-enantiomers (ESI† and Fig. 5).
The planes defined by the ortho-methylphenyl group and the 1,2-dihydro-1,3λ4,2λ4-diazaborinine ring in both 9-(rac) and 10-(rac) are close to orthogonal, with twist angles of 85.9 and 85.7 degrees, respectively, suggesting significant steric hindrance around the chiral axis.
10-(rac) was well resolved by semi-preparative chiral HPLC (Chiralpak AD-H, Heptane/IPA 85/15) giving sufficient of both enantiomers of 10 for further study (ESI†). Both enantiomers of 10 gave identical 1H NMR spectra to that of 10-(rac), whilst giving weak but opposite [α]D values of +13.0 and −13.0 respectively (henceforth labelled 10-(+) and 10-(−)).
To assign absolute configuration of the enantiomeric samples 10-(+) and 10-(−) vibrational circular dichroism (VCD), Raman optical activity (ROA) and electronic circular dichroism (ECD) measurements were performed. In agreement with DFT calculations, VCD spectroscopy showed no significant signals for 10-(+)/10-(−), preventing the use of VCD for absolute configuration determination (see ESI† for further discussion).18 Because of intense fluorescence, no useful ROA data could be measured;19 however, good experimental ECD spectra of both 10-(+) and 10-(−) were obtained in the range 175–500 nm using a Chirascan-plus spectrometer (Applied Photophysics Ltd).
Boltzmann-weighted ECD spectra for the postulated (R)-10 enantiomer were obtained from TD-DFT calculations at the cam-B3LYP/6-311++G(2d,p) level.20 First a low-energy conformation library was generated, followed by calculation of the individual ECD spectra for each of the low-energy conformations. The combined Boltzmann-weighted spectrum was then blue-shift corrected by 10 nm, to compensate for the typical underestimation of transition energies by TD-DFT (ESI†).20
Comparison of the corrected Boltzmann-weighted ECD spectrum obtained for (R)-10 showed that, for the near-UV, good agreement is obtained with the experimental ECD spectrum of 10-(+), whilst at longer wavelengths, the smaller ECD features are less well reproduced (Fig. 6). The agreement between experiment and theory in the 175–275 nm region allows the absolute stereochemistry of 10-(+) to be assigned as (R)-10-(+) and thus 10-(−) must have opposite stereochemistry, (S)-10-(−).
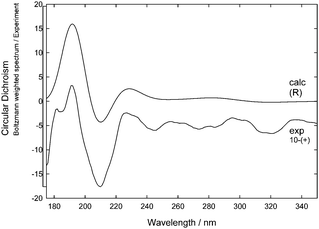 | ||
| Fig. 6 Comparison of calculated ECD spectra of (R)-10 [top] and experimentally measured ECD 10-(+) [bottom]. | ||
In conclusion, we have reported a synthetically flexible route to a class of axially chiral fluorophores (Ax*-BODIPYs), including resolution and absolute stereochemical determination by combined ECD/TD-DFT. Further research will focus on the interactions of Ax*-BODIPYs with chiral analytes in solution and applications to sensing.
The authors thank the Indonesian Ministry of National Education (R.I.L.) for funding, ESPRC for X-ray facilities at Newcastle University (EP/F03637X/1) and Dr Mike Probert (Newcastle) for crystallographic support, the EPSRC National Mass Spectrometry Service, Diamond Light Source for access to beamline I19, Bernard Costello, James Law and Dick Fielding (Applied Photophysics Ltd.) for ECD measurements and Prof. William McFarlane (Newcastle) for NMR support.
Notes and references
- A. Treibs and F.-H. Kreuzer, Justus Liebigs Ann. Chem., 1968, 718, 208 CrossRef CAS.
- A. Kamkaew, S. Hui Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77 RSC.
- (a) I. Johnson and M. T. Z. Spence, Molecular Probes Handbook, A Guide to Fluorescent Probes and Labeling Technologies, Life Technologies Ltd, 11th edn, 2010 Search PubMed; (b) A. Hendricks, E. J. Keliher, D. Wan, S. A. Hilderbrand, R. Weissleder and R. Mazitschek, Angew. Chem., Int. Ed., 2012, 51, 4603 CrossRef PubMed.
- N. Boens, V. Leena and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed; (b) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed.
- Y. Zhouab and J. Yoon, Chem. Soc. Rev., 2012, 41, 52 RSC.
- (a) F. Miao, J. Zhou, D. Tian and H. Li, Org. Lett., 2012, 14, 3572 CrossRef CAS PubMed; (b) G. Beer, K. Rurack and J. Daub, Chem. Commun., 2001, 1138 RSC; (c) J. Jiao, X. Liu, X. Mao, J. Li, Y. Cheng and C. Zhu, New J. Chem., 2013, 37, 317 RSC.
- D. Parker, R. S. Dickins, H. Puschmann, C. Crossland and J. A. K. Howard, Chem. Rev., 2002, 102, 1977 CrossRef CAS PubMed.
- (a) L. Pu, Chem. Rev., 2004, 104, 1687 CrossRef CAS PubMed; (b) H. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, K. Nobusawa, H. Tsumatori and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9266 CrossRef CAS PubMed; (c) Y. Sawada, S. Furumi, A. Takai, M. Takeuchi, K. Noguchi and K. Tanaka, J. Am. Chem. Soc., 2012, 134, 4080 CrossRef CAS PubMed.
- (a) G. Beer, C. Niederalt, S. Grimme and J. Daub, Angew. Chem., Int. Ed., 2000, 39, 3252 CrossRef CAS; (b) A. Gossauer, F. Nydegger, T. Kiss, R. Sleziak and H. Stoeckli-Evans, J. Am. Chem. Soc., 2004, 126, 1772 CrossRef CAS PubMed; (c) E. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, J. Bañuelos, T. Arbeloa, I. López-Arbeloa, M. J. Ortiz and S. de la Moya, Chem. Commun., 2013, 49, 11641 RSC; (d) E. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, M. J. Ortiz, B. G. Vo, G. Muller and S. de la Moya, J. Am. Chem. Soc., 2014, 136, 3346–3349 CrossRef PubMed.
- A. Haefele, C. Zedde, P. Retailleau, G. Ulrich and R. Ziessel, Org. Lett., 2010, 12, 1672 CrossRef CAS PubMed.
- (a) H. Kim, A. Burghart, M. B. Welch, J. Reibenspies and K. Burgess, Chem. Commun., 1999, 1889 RSC; (b) A. Loudet, R. Bandichhor, K. Burgess, A. Palma, S. O. McDonnell, M. J. Hall and D. F. O'Shea, Org. Lett., 2008, 10, 4771 CrossRef CAS PubMed; (c) C. Ikeda, T. Maruyama and T. Nabeshima, Tetrahedron Lett., 2009, 50, 3349 CrossRef CAS.
- (a) K. Yamada, T. Toyota, K. Takakura, M. Ishimaru and T. Sugawara, New J. Chem., 2001, 25, 667 RSC; (b) M. A. H. Alamiry, A. C. Benniston, J. Hagon, T. P. L. Winstanley, H. Lemmetyinen and N. V. Tkachenko, RSC Adv., 2012, 2, 4944 RSC; (c) H. L. Kee, C. Kirmaier, L. Yu, P. Thamyongkit, W. J. Youngblood, M. E. Calder, L. Ramos, B. C. Noll, D. F. Bocian, W. R. Scheidt, R. R. Birge, J. S. Lindsey and D. Holten, J. Phys. Chem. B, 2005, 109, 20433 CrossRef CAS PubMed.
- (a) M. K. Kuimova, G. Yahioglu, J. A. Levitt and K. Suhling, J. Am. Chem. Soc., 2008, 130, 6672 CrossRef CAS PubMed; (b) M. K. Kuimova, S. W. Botchway, A. W. Parker, M. Balaz, H. A. Collins, H. L. Anderson, K. Suhling and P. R. Ogilby, Nat. Chem., 2009, 1, 69 CrossRef CAS PubMed; (c) N. A. Hosny, G. Mohamedi, P. Rademeyer, J. Owen, Y. Wu, M.-X. Tang, R. J. Eckersley, E. Stride and M. K. Kuimovaa, PNAS, 2013, 110, 9225 CrossRef CAS PubMed; (d) M. A. H. Alamiry, A. C. Benniston, G. Copley, K. J. Elliott, A. Harriman, B. Stewart and Y.-G. Zhi, Chem. Mater., 2008, 20, 4024 CrossRef CAS; (e) A. C. Benniston, A. Harriman, V. Whittle and M. Zelzer, Eur. J. Org. Chem., 2010, 523 CrossRef CAS.
- During the preparation of this manuscript a related atropisomeric BODIPY oligomer system was reported: S. Kolemen, Y. Cakmak, Z. Kostereli and E. U. Akkaya, Org. Lett., 2014, 16, 660–663 CrossRef CAS PubMed.
- (a) A. C. Benniston, G. Copley, K. J. Elliott, R. W. Harrington and W. Clegg, Eur. J. Org. Chem., 2008, 2705 CrossRef CAS; (b) M. Bröring, R. Krüger, S. Link, C. Kleeberg, S. Köhler, X. Xie, B. Ventura and L. Flamigni, Chem. – Eur. J., 2008, 14, 2976 CrossRef PubMed; (c) A. C. Benniston, G. Copley, A. Harriman, D. Howgego, R. W. Harrington and W. Clegg, J. Org. Chem., 2010, 75, 2018 CrossRef CAS PubMed.
- gNMR V5, Adept Scientific plc, Letchworth Herts, UK, 2003 Search PubMed.
- Sampling for 72 hours and combination of the spectra of 10-(+) and 10-(−) for improved baseline correction using a BioTools ChiralIR-2X spectrometer gave no detectable VCD signals with a significant signal-to-noise ratio.
- ROA measurements were attempted using a BioTools ChiralRaman spectrometer.
- J. Autschbach, in Comprehensive Chiroptical Spectroscopy, Volume 1: Instrumentation, Methodology and Theoretical Simulations, ed. N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, John Wiley & Sons, 2012, ch. 21, p. 593 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV/Vis and fluorescence spectra for 8-(rac), 9-(rac) and 10-(rac), ECD spectra of 10-(+) and 10-(−), computational approaches towards predicted ECD and VCD spectra of 10-(+) and 10-(−), HPLC trace for the resolution of 10-(rac), experimental procedures for compounds 6–10, 1H and 13C spectra for compounds 6–10. Crystallographic data for 9-(rac) and 10-(rac). CCDC 984417 and 984418. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc00851k |
| This journal is © The Royal Society of Chemistry 2014 |