 Open Access Article
Open Access ArticleA rational approach for generating cardiac troponin I selective Spiegelmers†
Zsuzsanna
Szeitner
a,
Gergely
Lautner
b,
Szilvia K.
Nagy
a,
Róbert E.
Gyurcsányi
*b and
Tamás
Mészáros
*ac
aDepartment of Medical Chemistry, Molecular Biology and Pathobiochemistry, Semmelweis University, Tűzoltó u. 37-47, H-1094 Budapest, Hungary. E-mail: meszaros.tamas@med.semmelweis-univ.hu
bMTA-BME Chemical Nanosensors Research Group, Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Szent Gellért tér 4, H-1111 Budapest, Hungary. E-mail: robertgy@mail.bme.hu
cTechnical Analytical Research Group of HAS, Szent Gellért tér 4, H-1111 Budapest, Hungary
First published on 6th May 2014
Abstract
We report the first protein selective Spiegelmers of diagnostic relevance by rational identification of a target epitope and reverse screening of Spiegelmer candidates following the selection procedure. Application of the presented approach resulted in isolation of cardiac troponin I selective Spiegelmers with low nanomolar dissociation constant and functionality in serum.
Aptamers are oligonucleotide ligands that bind to their targets with high selectivity and affinity, rivaling the characteristics of antibody–antigen interactions.1 Furthermore, due to their in vitro selection procedure, entirely controlled chemical synthesis, and small size, aptamers are superior to antibodies in many terms, and thus have great potential for therapeutic and diagnostic applications.2,3 Despite the large number of innovative detection schemes, a breakthrough in practical and commercial exploitation of aptamers is still awaited. Hitherto, only mycotoxin and vascular endothelial growth factor selective aptamers have been marketed for food analysis and treatment of age-related macular degeneration, respectively.4,5 The development of the latter presents one of the main limitations for their application as therapeutic agents and diagnostic receptors. Namely, oligonucleotides are susceptible to the ubiquitously present nucleases, and thus rapidly degrade in the body fluids.6 Therefore, techniques have emerged for generation of aptamers built from modified nucleotides with enhanced nuclease resistance. Although different unnatural nucleotides have been successfully applied to increase the half-life of aptamers, none of them seems to be entirely nuclease resistant.7 To date, the only exceptions demonstrating seemingly complete resistance to enzymatic degradation are those composed of L-ribose or L-2′-deoxyribose units, i.e., Spiegelmers.8 These oligonucleotides are enantiomers of natural RNA and DNA molecules, possess the chemical properties of aptamers, but are unsusceptible to nuclease digestion. Generation of Spiegelmers is a more elaborate procedure than selection of aptamers and requires the equivalent mirror image of protein targets. Due to the limitations of peptide synthesis, production of the D-enantiomer of proteins is practically not feasible. Consequently, most of the reported Spiegelmers have been selected for cytokines and peptide hormones.9,10 Notwithstanding, considering that aptamers and Spiegelmers do not recognize their full protein targets but interact with them via definite amino acid motifs, protein selective Spiegelmers can be isolated without application of proteins.11 This approach follows the rationality of antibody generation by using peptides of identified epitopes of protein molecules. Feasibility of this method has been demonstrated by isolation of Spiegelmers for a bacterial enterotoxin using a 25-mer D-amino acid peptide.12 We developed this method further to produce Spiegelmers for cardiac troponin I (cTnI), one of the gold standard biomarkers of acute coronary syndrome (Fig. 1).
Although cTnI is released into the circulation upon myocardial injury, its detection is hampered by multiple factors among which are proteolytic degradation, phosphorylation, complexing with other molecules and cTnI-specific autoantibodies circulating in the blood of patients,13 as well as cross-reactivity of c-TnI receptors with skeletal muscle specific troponins (sTnIs).14 To alleviate these difficulties of troponin measurement, we carefully determined a 9-mer peptide hallmark sequence of cTnI. In comparison to sTnIs, cTnI possesses an N-terminal extension; hence, assigning an amino acid sequence from this region is expected to ensure the troponin I discriminating capacity of selected Spiegelmers.15 Although the N-terminal domain is an optimal choice in terms of selectivity of troponin I isoforms, both terminals of cTnI are extremely susceptible to proteolytic degradation; therefore, determination of the cTnI concentration using receptors that bind to the N-terminal of the protein could result in misleading data.16 Further important factors influencing accurate detection of cTnI are its interaction with troponin C and phosphorylation by protein kinase A.17 Considering these constraints of reliable troponin detection, we chose the epitope corresponding to positions 28–36 of cTnI. The D-enantiomer peptide of the assigned amino acid sequence was synthesized (D-cTnI peptide) and N-terminally extended using a cysteine to covalently link onto reactive bromoacetyl paramagnetic particles. Prior to selection of specific aptamers, the single-stranded DNA library of 1014 variants was challenged with unmodified paramagnetic beads to remove the nonspecifically interacting ssDNAs and obtain the initial selection library. The D-cTnI peptide specific oligonucleotides were isolated basically according to the previously described SELEX procedure by using the peptide-coated paramagnetic beads.18 The selection cycle was repeated nine times by gradually decreasing the peptide-coated particle concentration and incubation time combined with increased stringency of washing to enhance the affinity of selected ssDNAs (see ESI†). Following the last selection step, the PCR products were inserted into a cloning vector, transfected into competent cells, and the sequence of 85 inserts was determined by Sanger sequencing. Analysis of sequencing results indicated a successful selection by enrichment of the oligonucleotide library. Out of cloned inserts, 32 had the same sequence, 6 appeared twice, and the rest represented orphan sequences (Table S1, ESI†).
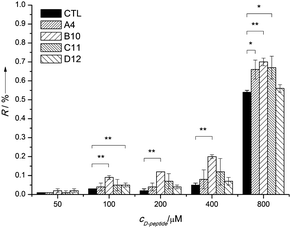
According to the theory of SELEX, the selection procedure results in enrichment of those aptamers that bind their target molecules with the highest affinity. However, it has also been reported that the selection could be distorted by intrinsic differences in the amplification efficiency of nucleic acid templates. Therefore, the most abundant oligonucleotides of SELEX do not necessarily represent the highest affinity aptamers.19 Consequently, in order to designate the most promising aptamers, the isolated oligonucleotides have to be evaluated individually in terms of their target-binding properties. To avoid the synthesis of each Spiegelmer candidate, we introduced a prescreening step with a reverse approach to identify the most promising Spiegelmers, i.e., to characterize the interaction of isolated D-oligonucleotides and D-cTnI peptide ligands by using surface plasmon resonance imaging (SPRi-HORIBA Jobin Yvon). Moreover, to evade the time-consuming and costly chemical synthesis of oligonucleotides, the evaluated D-oligonucleotides were produced by PCR using biotinylated forward and non-modified reverse primers of the selection library. The obtained fragments of the representative amplification reactions were microspotted using a solid contact pin onto ExtrAvidin coated SPR chips and directly converted into ssDNA by alkali denaturation for interaction analysis. Different concentration of D-cTnI peptide in selection buffer was flown over the sensor slides to estimate the affinity between selected oligonucleotides and D-cTnI peptides (Fig. 2).
According to these results, all studied oligonucleotides can bind the selected peptide ligand. Of note, the most abundant oligonucleotide (A4) demonstrated the second smallest affinity for the D-cTnI peptide affirming the previous finding that the most frequently represented sequence is not necessarily the best aptamer candidate.
To investigate whether the binding properties observed during prescreening of selected D-oligonucleotides using the mirrored cTnI epitope peptide are indicative of the Spiegelmer–protein binding, both the most promising (B10) and the most abundant (A4) sequences were synthesized from L-nucleotides with 5′ terminal thiol labelling. Next, the thiolated Spiegelmers were spotted onto bare gold sensor slides to characterize their cTnI protein binding kinetics by SPRi. The Spiegelmer spotted chips were blocked with (11-mercaptoundecyl)tetra(ethylene glycol) (HS-TEG),20 then incubated with various concentrations of the cTnI protein in selection buffer (Fig. 3).
The equilibrium dissociation constants of both Spiegelmers and the troponin protein were in the low nanomolar range (KD = 3.5 nM for B10 and KD = 10.7 nM for A4) and in agreement with the results of the D-DNA strand prescreening, the Spiegelmer equivalent of the most abundant oligonucleotide (A4) possessed a lower affinity towards the cTnI protein. Thus, the obtained data confirmed the results of the oligonucleotide screening experiment implying pertinence of the proposed reverse approach for identification of the most promising Spiegelmer candidates.
Having demonstrated the high cTnI affinity of selected Spiegelmers, we embarked on evaluating their selectivity. cTnI is a positively charged protein under physiological conditions (pI 10.31); thus, it could interact with the inherently negatively charged Spiegelmers by formation of non-selective, electrostatic interactions. To investigate this possibility, we measured the interaction of Spiegelmers with lysozyme (pI 11.35) – a protein notorious for non-specific binding –21 using the Spiegelmer spotted sensor slides (Fig. 4). No increase in the SPR signal was detected indicating that the electrostatic intermolecular forces between the Spiegelmer and cTnI are disrupted at the physiological ionic strength. The raised cTnI level in blood is a very specific indicator of cardiac muscle damage; however, false-positive values can be measured if the applied diagnostic receptor cross reacts with the closely homologous skeletal muscle troponin I (sTnI) isoforms. Although our target peptide of the selection procedure is specific for cTnI, we scrutinized the troponin I selectivity of Spiegelmers. To this end, the modified SPR sensor chips were challenged with purified sTnI. The measurements again revealed lack of interaction indicating that the analyzed Spiegelmers can discriminate the troponin I isoforms (Fig. 4).
Following cardiac cell death, the majority of cTnI is released into the circulation as a part of the ternary complex (cTnI–cTnT–TnC); therefore, the monomer and also the complex forming cTnI have to be detected to obtain diagnostically valuable data.16 To evaluate the cTnI complex perceiving capability of Spiegelmers, SPRi interaction analysis of Spiegelmer modified sensor chips with the commercial cTnI–cTnT–TnC protein complex was performed. The measurements showed obvious interactions with both Spiegelmers, and the SPR signals were higher than those measured with the monomer cTnI, which is in accordance with the larger molecular weight of the troponin complex (Fig. 3). These results suggest that the Spiegelmer probes detect both free and complex cTnI forms.
As a proof of their potential diagnostic receptor application, the Spiegelmers were tested for the selective recognition of cTnI in human blood serum. To this end, ten times diluted, troponin I free human serum was spiked with various amounts of cTnI to measure the cardiac marker protein concentration dependent alterations of the SPR signal. The results of the analysis showed a cTnI concentration dependent signal increase for both Spiegelmers, and in concert with the previous experiments, the most promising Spiegelmer (B10) demonstrated higher sensitivity for cardiac troponin I detection (Fig. 5).
Finally, to assess if the selected Spiegelmers meet the stability requirement of an ideal diagnostic receptor, the Spiegelmer spotted chip was desiccated and stored at 4 °C for six months, then challenged with cTnI. The SPRi measurements revealed no loss of sensitivity of the Spiegelmer modified sensor slides. Furthermore, the chips withstood multiple harsh regeneration steps using 20 mM NaOH, as shown by the unchanged SPR responses of subsequent cTnI measurements (Fig. S1, ESI†).
The in serum stability and analytical performance of the selected Spiegelmers imply that they have the potential to be used as cTnI specific receptors in cardiovascular diagnostic assays. Label-free SPR measurements are appropriate for kinetic characterization of affinity interactions, but they are not sensitive enough for analytical cTnI assays in the diagnostically relevant range (0.04–100 ng mL−1). However, what is important from the receptor development point of view is the KD of the Spiegelmer–cTnI complex, as the smaller this value the lower the expected LOD is. A thorough analysis of National Institute of Standards and Technology has revealed that the monoclonal antibodies of high sensitivity cTnI diagnostic kits possess KD values in the 74 nM–2.6 μM range, which are at least an order higher than that of our Spiegelmers.22 Therefore, it is expected that implementing a label-based amplification or a more sensitive detection technique, that is currently in development in our laboratories, the relevant diagnostic range of cTnI will be achievable. The superiority of the cTnI sensing capabilities of the Spiegelmer-modified chips is also highlighted by the fact that selective determination was achieved at low serum dilutions while conventional ELISA methods may need higher dilutions and a set of monoclonal antibodies to avoid matrix effects and infer selectivity.23
In summary, here we showed an approach indicating that protein selective Spiegelmers can be effectively produced by a rational identification of relevant protein epitopes combined with SPRi screening of isolated Spiegelmer candidates. The selection protocol resulted in isolation of a cardiovascular marker protein discriminatory Spiegelmers, which could be potentially applied in diagnostic assay development, as demonstrated by blood serum measurements and lack of cross-reactivity to sTnI.
The financial support from ENIAC (CAJAL4EU), the Momentum program of the Hungarian Academy of Sciences and of New Széchenyi Plan (TÁMOP-4.2.1./B-09/1/KMR-2010-0001) is gratefully acknowledged.
Notes and references
- E. N. Brody and L. Gold, J. Biotechnol., 2000, 74, 5–13 CAS.
- A. D. Keefe, S. Pai and A. Ellington, Nat. Rev. Drug Discovery, 2010, 9, 537–550 CrossRef CAS PubMed.
- E. J. Cho, J. W. Lee and A. D. Ellington, Annu. Rev. Anal. Chem., 2009, 2, 241–264 CrossRef CAS PubMed.
- J. Cruz-Toledo, M. McKeague, X. Zhang, A. Giamberardino, E. McConnell, T. Francis, M. C. Derosa and M. Dumontier, Database, 2012, 2012, bas006 CrossRef PubMed.
- E. W. Ng, D. T. Shima, P. Calias, E. T. Cunningham, Jr., D. R. Guyer and A. P. Adamis, Nat. Rev. Drug Discovery, 2006, 5, 123–132 CrossRef CAS PubMed.
- M. Famulok, G. Mayer and M. Blind, Acc. Chem. Res., 2000, 33, 591–599 CrossRef CAS PubMed.
- M. Kuwahara and N. Sugimoto, Molecules, 2010, 15, 5423–5444 CrossRef CAS PubMed.
- S. Klussmann, A. Nolte, R. Bald, V. A. Erdmann and J. P. Furste, Nat. Biotechnol., 1996, 14, 1112–1115 CrossRef CAS PubMed.
- K. Hoehlig, C. Maasch, N. Shushakova, K. Buchner, M. Huber-Lang, W. G. Purschke, A. Vater and S. Klussmann, Mol. Ther., 2013, 2, 2236–2246 CrossRef PubMed.
- F. Schwoebel, L. T. van Eijk, D. Zboralski, S. Sell, K. Buchner, C. Maasch, W. G. Purschke, M. Humphrey, S. Zollner, D. Eulberg, F. Morich, P. Pickkers and S. Klussmann, Blood, 2013, 121, 2311–2315 CrossRef CAS PubMed.
- V. J. Ruigrok, M. Levisson, J. Hekelaar, H. Smidt, B. W. Dijkstra and J. van der Oost, Int. J. Mol. Sci., 2012, 13, 10537–10552 CrossRef CAS PubMed.
- W. G. Purschke, F. Radtke, F. Kleinjung and S. Klussmann, Nucleic Acids Res., 2003, 31, 3027–3032 CrossRef CAS PubMed.
- S. Eriksson, M. Junikka, P. Laitinen, K. Majamaa-Voltti, H. Alfthan and K. Pettersson, Clin. Chem., 2003, 49, 1095–1104 CAS.
- P. J. O'Brien, Y. Landt and J. H. Ladenson, Clin. Chem., 1997, 43, 2333–2338 CAS.
- K. E. Hastings, Cell Struct. Funct., 1997, 22, 205–211 CrossRef CAS.
- A. G. Katrukha, A. V. Bereznikova, V. L. Filatov, T. V. Esakova, O. V. Kolosova, K. Pettersson, T. Lovgren, T. V. Bulargina, I. R. Trifonov, N. A. Gratsiansky, K. Pulkki, L. M. Voipio-Pulkki and N. B. Gusev, Clin. Chem., 1998, 44, 2433–2440 CAS.
- A. G. Katrukha, A. V. Bereznikova, T. V. Esakova, K. Pettersson, T. Lovgren, M. E. Severina, K. Pulkki, L. M. Vuopio-Pulkki and N. B. Gusev, Clin. Chem., 1997, 43, 1379–1385 CAS.
- M. B. Murphy, S. T. Fuller, P. M. Richardson and S. A. Doyle, Nucleic Acids Res., 2003, 31, e110 CrossRef PubMed.
- T. Schutze, B. Wilhelm, N. Greiner, H. Braun, F. Peter, M. Morl, V. A. Erdmann, H. Lehrach, Z. Konthur, M. Menger, P. F. Arndt and J. Glokler, PLoS One, 2011, 6, e29604 Search PubMed.
- G. Lautner, Z. Balogh, V. Bardóczy, T. Meszáros and R. E. Gyurcsányi, Analyst, 2010, 135, 918–926 RSC.
- J. Bognár, J. Szűcs, Z. Dorkó, V. Horváth and R. E. Gyurcsányi, Adv. Funct. Mater., 2013, 23, 4703–4709 CrossRef.
- M. S. Lowenthal, H. Gasca-Aragon, J. E. Schiel, N. G. Dodder and D. M. Bunk, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 2726–2732 CrossRef CAS PubMed.
- C. Heeschen, B. U. Goldmann, L. Langenbrink, G. Matschuck and C. W. Hamm, Clin. Chem., 1999, 45, 1789–1796 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the Spiegelmer selection and screening, SPRi measurements, and stability of the Spiegelmers. See DOI: 10.1039/c4cc00447g |
| This journal is © The Royal Society of Chemistry 2014 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: