Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid-state conversion of a MOF to a metal-organo polymeric framework (MOPF) via [2+2] cycloaddition reaction†
In-Hyeok
Park
a,
Anjana
Chanthapally
b,
Hyeong-Hwan
Lee
a,
Hong Sheng
Quah
b,
Shim Sung
Lee
*a and
Jagadese J.
Vittal
*ab
aDepartment of Chemistry and Research Institute of Natural Science, Gyeongsang National University, Jinju 660-701, S. Korea. E-mail: sslee@gnu.ac.kr; Fax: +82 55-753-7614; Tel: +82 55-772-1483
bDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543. E-mail: chmjjv@nus.edu.sg; Fax: +65 6779 1691; Tel: +65 6516 2975
First published on 23rd January 2014
Abstract
The bpeb ligands aligned in a slip-stacked manner in a two-fold interpenetrated non-porous metal–organic framework (MOF) [Zn2(bpeb)(bdc)(fa)2] undergo [2+2] cycloaddition reaction in a single-crystal to single-crystal manner to a non-interpenetrated 3D structure with a new topology comprising an organic polymer ligand and a 2D coordination polymer.
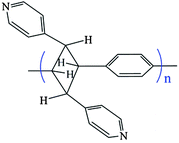
A number of highly crystalline organic polymers can be made in the solid state from a variety of monomers.1 In contrast, although organic polymers containing pendant metal complexes are known,2 their solid state structures could not be determined due to their very low crystallinity. The reason being, the traditional crystallization method may not be applicable to synthesize single crystals of metal complexes containing organo polymeric ligands. Recently we have observed that such a metal complex of an organic polymer ligand can be obtained indirectly by the [2+2] cycloaddition reaction in the solid state.3 The organic polymer containing cyclobutane rings has been incorporated into a metal–organic framework (MOF) using 1,4-bis[2-(4′-pyridyl)ethenyl]benzene (bpeb). This is possible because of the infinitely slip-staked conjugated C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the bpeb ligands forming a plane in the MOF. The photo-dimerization of these C
C bonds of the bpeb ligands forming a plane in the MOF. The photo-dimerization of these C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond pairs in the solid state under UV light polymerized these diolefins to furnish the desired metal-complex of the organic polymer ligand 1,3-(4,4′-bipyridyl)-2,4-phenylene-cyclobutane (poly-bppcb) as shown in Scheme 1.
C bond pairs in the solid state under UV light polymerized these diolefins to furnish the desired metal-complex of the organic polymer ligand 1,3-(4,4′-bipyridyl)-2,4-phenylene-cyclobutane (poly-bppcb) as shown in Scheme 1.
The product contains a 1D organo polymeric chain and a 1D coordination polymer fused together as metal-organo polymeric frameworks (MOPFs). The generality of the strategy for using [2+2] cycloaddition photo-polymerization reaction to make MOPF compounds should be established before MOPF compounds could be designed with desired properties for various applications. In addition, the success of this method will provide a better understanding for extending this strategy to make MOF–COF (covalent–organic framework) hybrid compounds which may have better properties of MOFs and COFs.
We have tested the hypothesis that an infinite head-to-tail arrangement of the bpeb ligands in a plane is required such that each C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the bpeb ligand is aligned to different adjacent spacer ligands to generate poly-bppcb using a new photoreactive MOF with cds topology. Interestingly, a doubly interpenetrated structure undergoes single-crystal to single-crystal (SCSC) transformation yielding the desired MOPF containing the organic polymer as depicted in Scheme 2. The details are given in this communication.
C bond in the bpeb ligand is aligned to different adjacent spacer ligands to generate poly-bppcb using a new photoreactive MOF with cds topology. Interestingly, a doubly interpenetrated structure undergoes single-crystal to single-crystal (SCSC) transformation yielding the desired MOPF containing the organic polymer as depicted in Scheme 2. The details are given in this communication.
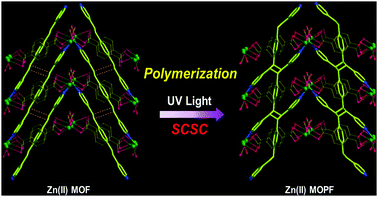 | ||
| Scheme 2 The SCSC transformation from MOF to MOPF by polymerization via [2+2] photo-cycloaddition reaction. | ||
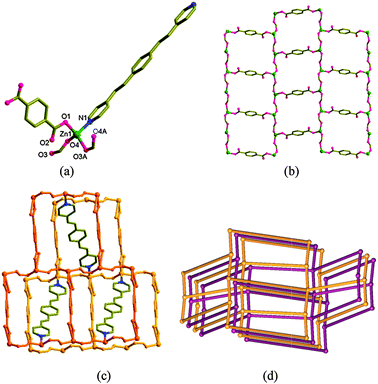
Orange block crystals of [Zn2(bpeb)(bdc)(fa)2] (1) suitable for single crystal X-ray data collection were obtained under solvothermal conditions from Zn(NO3)2·4H2O, 1,4-benzenedicarboxylic acid (H2bdc) and bpeb in a mixture of DMF (dimethylformamide) and water at 100 °C, followed by slow cooling. The origin of a formate ligand in the structure is due to the partial hydrolysis of DMF solvent under the experimental conditions.4 Interestingly, intentional use of formic acid or sodium formate in the synthesis did not yield 1. The purity of the bulk product was however confirmed by comparing the simulated powder X-ray diffraction (PXRD) pattern of the single crystal with that of the bulk sample (Fig. S1, ESI†). X-ray crystallographic experiments carried out at −100 °C revealed that the asymmetric unit contains only half of the atoms in the formula unit of 1.‡ The crystallographic inversion centre is present in the middle of bpeb and bdc ligands. Further the second formate ligand is related by the n-glide through the O3 atom (Fig. 1a). The distorted tetrahedral Zn1 [99.76(8)–124.65(8)°] is coordinated to one N atom of the bpeb spacer ligand, one O atom of the bdc ligand and O atoms of two formate ligands (Fig. 1a). The Zn1⋯O2 and Zn1⋯O4A distances, 2.77 and 2.90 Å, respectively, are considered very weak.
In 1, the Zn(II) atoms are bridged by the formate ligands forming a chain along the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01] direction and further cross-linked by a bdc spacer giving a highly corrugated brick-wall type [6,3] grid made from [Zn2(bdc)(fa)2] (Fig. 1b). The 3D coordination polymer is produced by the connectivity of the spacer ligand bpeb. Dictated by the tetrahedral geometry at Zn1 in the [Zn(fa)2] chain, only the alternate bpeb ligands point in the same direction as shown in Fig. 1c. Viewed along the a-axis, all these layers are well aligned leaving the bpeb ligands to occupy the channels. The resultant connectivity generated the cds topology in 1 (Fig. 1d) with Schlafli symbol {65·8}. The large void produced in this connectivity is filled by two-fold interpenetration (Fig. 1d) with little solvent accessible void.
01] direction and further cross-linked by a bdc spacer giving a highly corrugated brick-wall type [6,3] grid made from [Zn2(bdc)(fa)2] (Fig. 1b). The 3D coordination polymer is produced by the connectivity of the spacer ligand bpeb. Dictated by the tetrahedral geometry at Zn1 in the [Zn(fa)2] chain, only the alternate bpeb ligands point in the same direction as shown in Fig. 1c. Viewed along the a-axis, all these layers are well aligned leaving the bpeb ligands to occupy the channels. The resultant connectivity generated the cds topology in 1 (Fig. 1d) with Schlafli symbol {65·8}. The large void produced in this connectivity is filled by two-fold interpenetration (Fig. 1d) with little solvent accessible void.
The relative orientations of the bpeb ligands in the interpenetrated structures make 1 photoreactive. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the neighbouring bpeb ligands are infinitely arranged closely in a slip-stacked manner as shown in Fig. 2. The phenylene rings are closer to the adjacent pyridyl groups (centroid to centroid distance of 3.616 Å) showing face-to-face π–π interactions. This makes only each C
C bonds in the neighbouring bpeb ligands are infinitely arranged closely in a slip-stacked manner as shown in Fig. 2. The phenylene rings are closer to the adjacent pyridyl groups (centroid to centroid distance of 3.616 Å) showing face-to-face π–π interactions. This makes only each C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in a given spacer ligand is aligned to two different neighbouring ligands and the distance between the centres of C
C bond in a given spacer ligand is aligned to two different neighbouring ligands and the distance between the centres of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds is 3.559 Å. The [2+2] cycloaddition reaction of 1 under UV light is expected to generate an organic polymer based on cyclobutane rings fused with the [Zn2(bdc)(fa)2] layers forming an interesting MOPF structure.
C bonds is 3.559 Å. The [2+2] cycloaddition reaction of 1 under UV light is expected to generate an organic polymer based on cyclobutane rings fused with the [Zn2(bdc)(fa)2] layers forming an interesting MOPF structure.
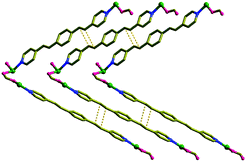 | ||
| Fig. 2 A slip-stacked alignment of the neighbouring bpeb ligands in 1 showing only the relevant atoms. | ||
Irradiation of the orange single crystals of 1 under a Xe-lamp of wavelength 365 nm for 2 h resulted in pale yellow broken crystals, but a single crystal suitable for the single crystal X-ray analysis was found.‡ Further characterisation by routine solution 1H-NMR spectroscopy was not possible due to its insolubility even in strong acids, indirectly inferring that the expected organic polymer has formed. This behaviour is very similar to that reported recently.3 Moreover, the single crystals of 1 are also insoluble even when trying to digest using strong acids.
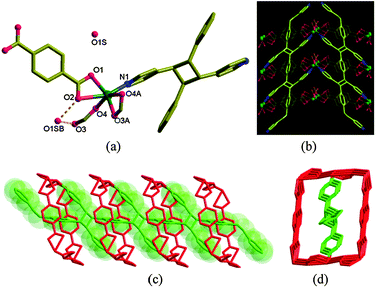
The X-ray crystallographic analysis of [Zn2(poly-bppcb)(bdc)(fa)2]·H2O (2) revealed that the quantitative photo-cyclization accompanied by structural transformation has occurred (Fig. 3). Although a different space group setting is used (P21/n in 1 and to P21/c in 2), 2 is essentially isomorphous to 1. Due to an increase in the volume of the unit cell (1321.91(4) Å3 in 1 to 1433.1(5) Å3 in 2), the crystals take up a water molecule from air during the experiment as revealed in the crystal structure (Fig. S11, ESI†). As might be expected, the water molecule (O1SB) is stabilised by the H-bonds bound to the oxygen donors (O2 and O3) from bdc and fa (dashed lines in Fig. 3a). The same crystallographic symmetries are present in 2 except that the c-glide is present along the [Zn(fa)2] chain. Due to the poly-bppcb ligand in 2, the coordination environment at Zn1 is perturbed from a highly distorted tetrahedral geometry in 1 to a highly distorted octahedral geometry with both carboxylates chelating the Zn1 atom.
Due to the formation of cyclobutane rings between the cds structures that resulted in the new polymer ligand, poly-bppcb (Fig. 3b), the original two-fold interpenetrated net in 1 is now fused into a single net in 2 (Fig. 3c and d). The organic ligand, poly-bppcb, is enclosed inside the channels formed by the [Zn(bdc)(fa)2] brick-wall layers along the a-axis and is cross-linked with the ZnII atoms through pyridyl N atoms.
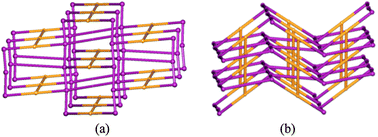
This SCSC photo-polymerization of the bpeb ligands by [2+2] cycloaddition reactions causes the structural transformation of a doubly-interpenetrated non-porous MOF with cds topology to a non-interpenetrating network with a new topology. This unusual binodal net is built from the tetrahedral nodes of Zn(II) atoms and square planar nodes created by the cyclobutane rings of the poly-bppcb ligand. It is this infinite extension of the ligand which results in a new (4, 4) connected net which we call jjv2 with point symbol {62.83.10}{65.8}2 and vertex symbol [6.62.6.62.6.84] [62.62.85.85.86.1018] as shown in Fig. 4. Such a polyfused [4+4] grid in coordination polymers can result in new or rare topologies.3
Solid state photoluminescence spectra were recorded for 1 and 2 and were compared with that of the bpeb ligand. The free ligand is weakly emissive in the yellow region, λmax = 558 nm when excited at λex = 360 nm. Compound 1 shows a strong green emission with a λmax at 507 nm while 2 has a weaker emission which is blue shifted to more strong blue emission at λmax = 429 nm which might be due to the loss of extended conjugation upon polymerization (Fig. S7 and S8, ESI†).
In this work an interesting photoreactive non-porous MOF containing two different carboxylate spacer ligands was synthesized. The formate anion could only be incorporated into this MOF indirectly by the partial hydrolysis of the DMF. The bpeb ligands have been aligned in a slip-stacked manner in a plane such that each C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the bpeb ligand is aligned to different adjacent spacer ligands. Hence photochemical [2+2] cycloaddition reaction leads to the polymerization of the conjugated dienes in the bpeb ligands via [2+2] cycloaddition reaction. The infinite slip-stacked alignment has been achieved by the tetrahedral Zn(II) atoms in the cds topology instead of a diamondoid network as reported earlier.3 The original two-fold interpenetrated cds structure is transformed under UV light to a non-interpenetrated structure jjv2, with a new topology, in a SCSC manner due to the formation of cyclobutane rings between the adjacent C
C bond in the bpeb ligand is aligned to different adjacent spacer ligands. Hence photochemical [2+2] cycloaddition reaction leads to the polymerization of the conjugated dienes in the bpeb ligands via [2+2] cycloaddition reaction. The infinite slip-stacked alignment has been achieved by the tetrahedral Zn(II) atoms in the cds topology instead of a diamondoid network as reported earlier.3 The original two-fold interpenetrated cds structure is transformed under UV light to a non-interpenetrated structure jjv2, with a new topology, in a SCSC manner due to the formation of cyclobutane rings between the adjacent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. This 3D structure has a 2D coordination polymer [Zn2(bdc)(fa)2] fused together with an organic polymer, poly-bppcb, to give a new MOPF compound which is unlikely to be synthesized by any other means. We are currently exploring the possibility of extending this strategy to synthesize MOF–COF hybrid structures which are expected to exhibit interesting sorption properties.
C bonds. This 3D structure has a 2D coordination polymer [Zn2(bdc)(fa)2] fused together with an organic polymer, poly-bppcb, to give a new MOPF compound which is unlikely to be synthesized by any other means. We are currently exploring the possibility of extending this strategy to synthesize MOF–COF hybrid structures which are expected to exhibit interesting sorption properties.
This work was supported by the NRF (2012R1A4A1027750), S. Korea, and the Ministry of Education, Singapore, through NUS FRC grant R-143-000-562-112.
Notes and references
- (a) F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570–4571 CrossRef CAS PubMed; (b) S. Rondeau-Gagné, J. R. Néabo, M. Desroches, J. Larouche, J. Brisson and J.-F. Morin, J. Am. Chem. Soc., 2013, 135, 110–113 CrossRef PubMed; (c) A. Matsumoto, D. Furukawa, Y. Mori, T. Tanaka and K. Oka, Cryst. Growth Des., 2007, 7, 1078–1085 CrossRef CAS; (d) X. Ouyang, F. W. Fowler and J. W. Lauher, J. Am. Chem. Soc., 2003, 125, 12400–12401 CrossRef CAS PubMed; (e) A. Matsumoto and T. Odani, Macromol. Rapid Commun., 2001, 22, 1195–1215 CrossRef CAS; (f) R. Xu, V. Gramlich and H. Frauenrath, J. Am. Chem. Soc., 2006, 128, 5541–5547 CrossRef CAS PubMed; (g) H. Peng, J. Tang, J. Pang, D. Chen, L. Yang, H. S. Ashbaugh, C. J. Brinker, Z. Yang and Y. Lu, J. Am. Chem. Soc., 2005, 127, 12782–12783 CrossRef CAS PubMed; (h) T. Itoh, T. Shichi, T. Yui and K. Takagi, Langmuir, 2005, 21, 3217–3220 CrossRef CAS PubMed; (i) S. M. Curtis, N. Le, F. W. Fowler and J. W. Lauher, Cryst. Growth Des., 2005, 5, 2313–2321 CrossRef CAS; (j) K. Morigaki, K. Kiyosue and T. Taguchi, Langmuir, 2004, 20, 7729–7735 CrossRef CAS PubMed; (k) S. B. Lee, R. Koepsel, D. B. Stolz, H. E. Warriner and A. J. Russell, J. Am. Chem. Soc., 2004, 126, 13400–13405 CrossRef CAS PubMed; (l) G. W. Coate, A. R. Dunn, L. M. Henling, J. W. Ziller, E. B. Lobkovsky and R. H. Grubbs, J. Am. Chem. Soc., 1998, 120, 3641–3649 CrossRef; (m) M. Hasegawa, in Adv. Phys. Org. Chem., ed. D. Bethell, Academic Press, 1995, vol. 30, pp. 117–171 Search PubMed; (n) J. W. Lauher, F. W. Fowler and N. S. Goroff, Acc. Chem. Res., 2008, 41, 1215–1229 CrossRef CAS PubMed; (o) T. Hoang, J. W. Lauher and F. W. Fowler, J. Am. Chem. Soc., 2002, 124, 10656–10657 CrossRef CAS PubMed; (p) J. Xiao, M. Yang, J. W. Lauher and F. W. Fowler, Angew. Chem., Int. Ed., 2000, 39, 2132–2135 CrossRef CAS; (q) S. Nomura, T. Itoh, H. Nakasho, T. Uno, M. Kubo, K. Sada, K. Inoue and M. Miyata, J. Am. Chem. Soc., 2004, 126, 2035–2041 CrossRef CAS PubMed; (r) M. Hasegawa, Pure Appl. Chem., 1986, 58, 1179–1188 CrossRef CAS; (s) M. Hasegawa, Chem. Rev., 1983, 83, 507–518 CrossRef CAS; (t) L. Addadi and M. Lahav, J. Am. Chem. Soc., 1978, 100, 2838–2844 CrossRef CAS.
- (a) J. M. Pollino and M. Weck, Chem. Soc. Rev., 2005, 34, 193–207 RSC; (b) M. O. Wolf, J. Inorg. Organomet. Polym. Mater., 2006, 16, 189–199 CrossRef CAS; (c) T. Kaliyappan and P. Kannan, Prog. Polym. Sci., 2000, 25, 343–370 CrossRef CAS.
- I.-H. Park, A. Chanthapally, Z. Zhang, S. S. Lee, M. J. Zaworotko and J. J. Vittal, Angew. Chem., Int. Ed., 2014, 53, 414–419 CrossRef CAS PubMed.
- (a) Q. Sun, Q. Yue, J.-Y. Zhang, L. Wang, X. Li and E.-Q. Gao, Cryst. Growth Des., 2009, 9, 2310–2317 CrossRef CAS; (b) F. Dai, D. Sun and D. Sun, Cryst. Growth Des., 2011, 11, 5670–5675 CrossRef CAS; (c) A. D. Burrows, K. Cassar, R. M. W. Friecnd, M. F. Mahon, S. P. Rigby and J. E. Warren, CrystEngComm, 2005, 7, 548 RSC; (d) J. He, J. Yu, Y. Zhang, Q. Pan and R. Xu, Inorg. Chem., 2005, 44, 9279 CrossRef CAS PubMed; (e) X. L. Wang, L. Gan, S. W. Zhang and S. Gao, Inorg. Chem., 2004, 43, 4615 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: XRPD patterns, TGA curves, solid-state photographs and crystal structures. CCDC 955835 (1) and 955836 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc00228h |
| ‡ Crystal data for 1: C30H22N2O8Zn2, fw = 669.24, monoclinic, P21/n, a = 6.8414(1) Å, b = 21.8416(4) Å, c = 9.4560(2) Å, β = 110.684(1)°, V = 1321.91(4) Å3, Z = 2, Dx = 1.681 g cm−3, μ = 1.873 mm−1, Rint = 0.0311, GOF = 1.097, R1 = 0.0316, wR2 = 0.0781 for 2424 data I > 2σ(I). Crystal data for 2: C30H24N2O9Zn2, fw = 687.26, monoclinic, P21/c, a = 7.3685(18) Å, b = 22.092(4) Å, c = 9.448(2) Å, β = 111.283(10)°, V = 1433.1(5) Å3, Z = 2, Dx = 1.593 g cm−3, μ = 1.732 mm−1, Rint = 0.1037, GOF = 1.047, R1 = 0.0672, wR2 = 0.1487 for 1895 data I > 2σ(I). |
| This journal is © The Royal Society of Chemistry 2014 |