Hui Shao, Xiao-Ming Zhang, Shao-Hua Wang, Fu-Min Zhang, Yong-Qiang Tu and Chao Yang
Chem. Commun., 2014,50, 5691-5694
DOI:
10.1039/C3CC49650C,
Communication
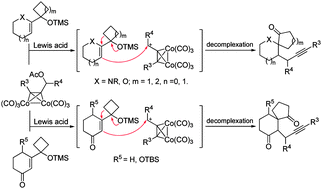
A novel propargylic electrophile-induced tandem intermolecular addition–semipinacol rearrangement was developed efficiently under mild conditions. Various allylic silylether substrates as well as Co-complexed propargylic species were applicable to this protocol and gave a series of synthetically useful β-propargyl spirocyclic ketones in moderate to good yields. Its synthetic application was also demonstrated by an efficient construction of the key tricyclic moiety of daphlongamine E.