Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Outer-sphere anion recognition by a cyclen-based octadentate europium(III) complex: pH dependent recognition of ortho-phthalic acid†
Desigan
Sannasy
,
Helder M.
Marques
,
Manuel A.
Fernandes
and
Alvaro S.
de Sousa
*
Molecular Sciences Institute, School of Chemistry, University of the Witwatersrand, P. O. Wits, Johannesburg, 2050 South Africa. E-mail: alvaro.desousa@wits.ac.za; Fax: +27 11 717 6749; Tel: +27 11 717 6710
First published on 18th December 2013
Abstract
An octadentate cyclen-based europium complex with amidic and hydroxyalkyl pendent moieties exhibits pH dependent ligand denticity associated with anion recognition. Unusually high hydration numbers are determined for ortho-phthalate ternary outer-sphere complexes for which modulation of lanthanide-based luminescence is observed.
The design of chelating anion sensors using cyclen-based europium complexes has focussed upon heptadentate cyclen chelates bearing three pendent side-arms. In these complexes the europium square-antiprismatic coordination polyhedron includes two aqua ligands. Anion recognition events require selective displacement of aqua ligands upon binding of the anion to the metal centre. Biologically relevant dicarboxylate anions form five- or six-membered chelate rings upon binding to the metal centre with concomitant modulation of sensitised lanthanide emission.1 However, recognition of aromatic carboxylate anions is reported to be more effective using terbium(III) analogues.2
Enhanced luminescence has been observed upon binding of the N,N-dimethylaminocarboxylate anion to a heptadentate cationic terbium(III) complex at physiological pH.2 The aromatic carboxylate acts as a sensitising antenna capable of adequately populating the 5D4 Tb(III) excited state of the ternary complex, while simultaneously displacing coordinated water molecules that quench the lanthanide emission, thereby signalling the presence of the anion. Hydration states determined using a Horrocks-based method,3 endorse bidentate carboxylate binding to form four-membered chelate rings, displacing inner-sphere water molecules. Consequently, sensing of aliphatic and aromatic dicarboxylates was further investigated using dinuclear terbium complexes.3 Dinuclear complexes encapsulated terbium(III) ions within a cyclen macrocycle bearing three pendent amide moieties, covalently linked via a p-xylyl spacer aimed at accommodating the binding requirements of the dicarboxylate anions. Each metal centre was shown to independently bind a N,N-dimethylaminocarboxylate anion in dinuclear ternary complexes with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. A 1
1 stoichiometry. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was observed for the luminescent ternary complex of terephthalic acid in which carboxylate moieties bind to separate metal centres forming four-membered chelate rings. A number of phthalate derivatives have been identified as metabolic and endocrine disruptors, capable of inducing hormone imbalance in humans with highly toxic effects.4–9 Recognition of aromatic dicarboxylates is therefore desirable in biological and aqueous media. Hexaazamacrocyclic ligands consisting of amines linked by p-xylyl spacers and alkyl groups are reported to afford discriminatory recognition of phthalate isomers.10 Potentiometrically determined formation constants for phthalate ternary complexes with hexaazamacrocycles containing propylene groups, indicate recognition of the isophthalate complex is thermodynamically preferred. Inappropriate geometries of the ortho and para isomers inhibit optimal N–H⋯O and C–H⋯O hydrogen-bonding interactions with the terephthalate (para) complex exhibiting the weakest binding.
1 stoichiometry was observed for the luminescent ternary complex of terephthalic acid in which carboxylate moieties bind to separate metal centres forming four-membered chelate rings. A number of phthalate derivatives have been identified as metabolic and endocrine disruptors, capable of inducing hormone imbalance in humans with highly toxic effects.4–9 Recognition of aromatic dicarboxylates is therefore desirable in biological and aqueous media. Hexaazamacrocyclic ligands consisting of amines linked by p-xylyl spacers and alkyl groups are reported to afford discriminatory recognition of phthalate isomers.10 Potentiometrically determined formation constants for phthalate ternary complexes with hexaazamacrocycles containing propylene groups, indicate recognition of the isophthalate complex is thermodynamically preferred. Inappropriate geometries of the ortho and para isomers inhibit optimal N–H⋯O and C–H⋯O hydrogen-bonding interactions with the terephthalate (para) complex exhibiting the weakest binding.
Europium(III) complexes of coordinatively saturated cyclen-based ligands, bearing four pendent side-arms, have been thoroughly scrutinised; their coordination polyhedra inevitably include a singular axially bound aqua ligand undergoing dissociative exchange with bulk water. The aqua ligand is, however, not readily substituted in aqueous media11,12 and anion sensing strategies infrequently consider octadentate europium(III) complexes. Unsaturated coordination has recently been reported for octadentate europium(III) complexes in which a brachial moiety is not bound to the metal centre.13,14 A pendent azaxanthanone group of a protein-bound octadentate europium(III) complex is reported to reside in the apolar binding cavity of the alpha-1-glycoprotein and is not coordinated to the metal centre.13 A change in ligand denticity may also be advanced for preferential citrate recognition by Eu(S-THP)(H2O), a cyclen-based octadentate europium(III) complex bearing four brachial hydroxypropyl moieties.14 Displacement of the weaker hydroxyalkyl donors, in addition to the axially coordinated water molecule, facilitates a multidentate chelating mode for the ternary citrate complex exhibiting enhanced emission upon direct excitation.
In this work a mixed donor cyclen-based europium(III) complex is shown to exhibit fluxional ligand denticity upon phthalate recognition. The formation of a ternary ortho-phthalate complex is investigated by monitoring pH-dependent modulation of the characteristic europium(III) emission. Emission spectra were recorded for pH titrations in aqueous and buffered media, using biologically suited buffers (1 mM MOPS, 1 mM MES, 2.4 mM Tris, 1 mM KHP), at constant ionic strength. Hydration states, at relevant pH values are determined using a modified Horrocks method15,16 to account for quenching contributions of the hydroxycycloalkyl and methylethanamide pendents.
Endo conformations, resembling conformations in the structure of DTMA,17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‡ prevail for trans methylethanamide and the hydroxycycloalkyl pendents (ESI;† Fig. S1). The hydroxycycloalkyl pendent accommodates O–H⋯O hydrogen-bonding to a lattice water molecule separated from the macrocyclic N4 centroid by 4.45(1) Å (ESI;† Fig. S2 and Table S4). Outer-sphere water molecules, at comparable distances from the metal centre in cyclen-based europium(III) complexes, modestly quench (approximately 8% efficiency) lanthanide emission,18 suggesting O–H⋯O hydrogen bonding between the hydroxycyclohexyl pendent and proximal water molecules may only marginally influence lanthanide emission of the [Eu(CyD3MA)]3+ complex in aqueous solution.§
‡ prevail for trans methylethanamide and the hydroxycycloalkyl pendents (ESI;† Fig. S1). The hydroxycycloalkyl pendent accommodates O–H⋯O hydrogen-bonding to a lattice water molecule separated from the macrocyclic N4 centroid by 4.45(1) Å (ESI;† Fig. S2 and Table S4). Outer-sphere water molecules, at comparable distances from the metal centre in cyclen-based europium(III) complexes, modestly quench (approximately 8% efficiency) lanthanide emission,18 suggesting O–H⋯O hydrogen bonding between the hydroxycyclohexyl pendent and proximal water molecules may only marginally influence lanthanide emission of the [Eu(CyD3MA)]3+ complex in aqueous solution.§
First order luminescence decay in H2O and D2O indicates a single water molecule (q ∼ 1) is bound to the metal centre in the [Eu(CyD3MA)]3+ complex (ESI,† Table S1) at low pH.
The capped square-antiprism coordination geometry observed for the [Eu(CyD3MA)(CF3SO3)]2+ complex, as determined by single crystal X-ray crystallography (Fig. 1; ESI,† Table S5), shows a triflato ligand replacing the apically coordinated water molecule in the solid state. The Eu–O3SCF3 distance (2.443(1) Å; ESI,† Table S2) compares favourably with the average axial Eu–O distance (2.442 Å) of coordinated water molecules in monoaqua, nine-coordinate europium complexes.18 Weaker hydroxycycloalkyl coordination (Eu–O distance 2.408(1) Å), compared to amidic counterparts (Fig. 1; ESI,† Table S2), accommodates the apical metal–triflate interaction sterically unaffected by the brachial hydroxycyclohexyl moiety.
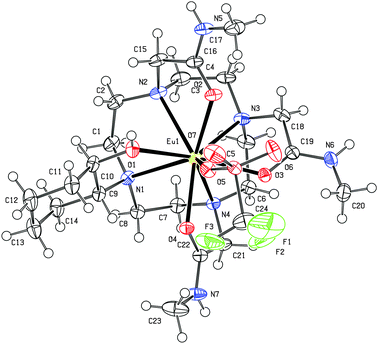 | ||
| Fig. 1 Ortep diagram of [Eu(CyD3MA)(CF3SO3)]2+. Thermal ellipsoids are shown at the 50% probability level. | ||
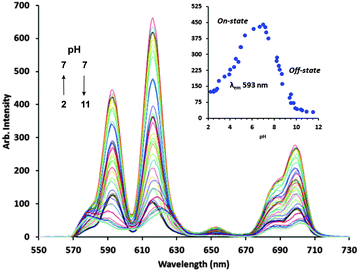
At elevated pH modulation of the europium(III) emission (detector PMT accelerator voltage 800 V) coincides with a decrease in the value of q (∼0.5) for [Eu(CyD3MA)(OH)]2+ that signals deprotonation of the aqua ligand (pKa = 8.7 ± 0.1; ESI,† Fig. S3 and S4).¶ The pKa deviates significantly from that observed for [Eu(DTMA)(OH)]2+ (pKa = 7.3);19 this is attributed to hydrogen-bonding interactions involving the hydroxycyclohexyl pendent and/or outer-sphere water molecules alluded to earlier.
Changes in hydration state (ESI,† Table S3), with concomitant pH dependent modulation of Eu(III) emission (Fig. 2 and Fig. S5, ESI†), are likewise observed for a multicomponent buffer solution (KHP, MOPS, MES and TRIS) of [Eu(CyD3MA)]3+. The spectroscopic signature for the buffered [Eu(CyD3MA)]3+ solution is influenced by the formation of an ortho-phthalate ternary complex with unusually high values of q for an octadentate europium(III) complex below physiological pH (ESI,† Table S3). Hydration states at lower pH support ligand heptadenticity upon formation of an outer-sphere [Eu(CyD3MA)(H2O)2]3+pht2− ternary complex, in which O4 equatorial binding of the hydroxycyclohexyl pendent is replaced by a second bound water molecule. pKa values of 4.7 ± 0.1 and 8.7 ± 0.1 are determined by non-linear regression analysis of luminescent data. Electronic absorption data (π–π* transition, 280 nm; pKa1 = 2.8 ± 0.2, pKa2 = 5.1 ± 0.1) confirms complete ortho-phthalate deprotonation in the ternary complex [Eu(CyD3MA)(H2O)2]3+ pht2− (ESI,† Fig. S6 and S7). O–H⋯Opht hydrogen-bonding interactions with inner-sphere water molecules and the uncoordinated hydroxycyclohexyl pendent20 facilitate outer-sphere phthalate binding with subsequent energy transfer from the phthalate (ortho) antennae to the europium metal centre – sensitising europium(III) emission. Deprotonation of the coordinated water (pKa = 8.7) leads to weaker electrostatic attraction in the ternary [Eu(CyD3MA)(H2O)OH]2+ pht2− complex, evidenced by a decrease in intensity for all Eu(III) emission bands (the Off-state). Phthalate displacement precedes hydroxycycloalkyl binding that results in the formation of the octadentate [Eu(CyD3MA)OH]2+ complex, verified by the determined hydration state (q ∼ 0.5) at elevated pH (ESI,† Table S3). Modulation of europium(III) emission is not observed below physiological pH for a buffered [Eu(CyD3MA)]3+ solution that excludes KHP (ESI,† Fig. S8). A single pKa (9.0 ± 0.1), determined from analysis of luminescence data (ESI,† Fig. S9), compares favourably with that observed for [Eu(CyD3MA)(H2O)]3+ (pKa = 8.7) in aqueous solution, further confirming that modulation of lanthanide emission at lower pH is phthalate induced.
This study identifies mixed donor octadentate europium(III) complexes as potential alternatives for pH dependent recognition of anions with suitable spectroscopic signatures. Spectroscopically silent octadentate europium(III) complexes are generally considered inappropriate for recognition of chelating anions. Necessary pendent donor displacement for inner-sphere coordination, potentially compromises complex stability. This work demonstrates formation of ternary outer-sphere complexes of anions, capable of sensitising europium-based emission, provides an alternative recognition event associated with changes in hydration state and ligand denticity. In particular, ortho-phthalate recognition by a mixed donor octadentate europium(III) complex, [Eu(CyD3MA)]3+, is illustrated. The ortho-phthalate anion is shown to be an outer-sphere ligand and binding interactions result in hydration states (q ∼ 2) more readily associated with heptadentate chelators. Pseudo-heptadenticity is achieved by incorporating a weaker binding brachial hydroxycycloalkyl group, susceptible to dissociation upon formation of an outer-sphere ternary complex. At higher pH values octadenticity is restored, as determined by hydration states characteristic of octadentate europium(III) complexes, upon ortho-phthalate displacement. The above mentioned responsive nature of mixed donor, octadentate europium(III) complexes merits further investigation that may potentially impact developments in anion and molecular recognition. Observations can further contribute towards the development of analogous responsive gadolinium(III) complexes suitable for applications in MRI imaging.
This work was supported by the University of the Witwatersrand, the National Research Foundation, and Sasol (South Africa).
Notes and references
- R. S. Dickins, S. Aime, A. S. Batsanov, A. Beeby, M. Botta, J. I. Bruce, J. A. K. Howard, C. S. Love, D. Parker, R. D. Peacock and H. Puschmann, J. Am. Chem. Soc., 2002, 124, 12697–12705 CrossRef CAS PubMed.
- T. Gunnlaugsson, A. J. Harte, J. P. Leonard and M. Nieuwenhuyzen, Supramol. Chem., 2003, 15, 505–519 CrossRef CAS.
- A. J. Harte, P. Jensen, S. E. Plush, P. E. Kruger and T. Gunnlaugsson, Inorg. Chem., 2006, 45, 9465–9474 CrossRef CAS PubMed.
- E. E. Hatch, J. W. Nelson, R. W. Stahlhut and T. F. Webster, Int. J. Androl., 2010, 33, 324–332 CrossRef CAS PubMed.
- J. N. Feige, L. Gelman, D. Rossi, V. Goete, R. Metivier, C. Tudor, S. I. Anghel, A. Grosdidier, C. Lathion, Y. Engelborghs, O. Michielin, W. Wahli and B. Desvergne, J. Biol. Chem., 2007, 282, 19152–19166 CrossRef CAS PubMed.
- C. Casals-Casas and B. Desvergne, Annu. Rev. Physiol., 2011, 73, 135–162 CrossRef CAS PubMed.
- A. Eveillard, F. Lasserre, M. de Tayrac, A. Polizzi, S. Claus, C. Canlet, L. Mselli-Lakhal, G. Gotardi, A. Paris, H. Guillon, P. G. P. Martin and T. Pineau, Toxicol. Appl. Pharmacol., 2009, 236, 282–292 CrossRef CAS PubMed.
- T. Fukuwatari, S. Ohsaki, S.-I. Kukuoka, R. Sasaki and K. Shibata, Toxicol. Sci., 2004, 81, 302–308 CrossRef CAS PubMed.
- B. Desvergne, J. N. Feige and C. Casals-Casas, Mol. Cell. Endocrinol., 2009, 304, 43–48 CrossRef CAS PubMed.
- A. Arbuse, C. Anda, M. A. Martinez, J. Perez-Miron, C. Jaime, T. Parella and A. Llobet, Inorg. Chem., 2007, 46, 10632–10638 CrossRef CAS PubMed.
- S. Aime, A. Barge, M. Botta, D. Parker and A. S. De Sousa, J. Am. Chem. Soc., 1997, 119, 4767–4768 CrossRef CAS.
- S. Aime, M. Botta, M. Fasano, M. P. M. Marques, C. F. G. C. Geraldes, D. Pubanz and A. E. Merbach, Inorg. Chem., 1997, 36, 2059–2068 CrossRef CAS PubMed.
- R. Carr, L. Di Bari, S. L. Piano, D. Parker, R. D. Peacock and J. M. Sanderson, Dalton Trans., 2012, 41, 13154–13158 RSC.
- J. Hammell, L. Buttarazzi, C.-H. Huang and J. R. Morrow, Inorg. Chem., 2011, 50, 4857–4867 CrossRef CAS PubMed.
- R. M. Supkowski and W. D. Horrocks Jr., Inorg. Chim. Acta, 2002, 340, 44–48 CrossRef CAS.
- R. M. Supkowski and W. D. Horrocks Jr., Inorg. Chem., 1999, 38, 5616–5619 CrossRef CAS PubMed.
- S. Aime, A. Barge, J. I. Bruce, M. Botta, J. A. K. Howard, J. M. Moloney, D. Parker, A. S. De Sousa and M. Woods, J. Am. Chem. Soc., 1999, 121, 5762–5771 CrossRef CAS.
- D. Parker, R. S. Dickins, H. Puschmann, C. Crossland and J. A. K. Howard, Chem. Rev., 2002, 102, 1977–2010 CrossRef CAS PubMed.
- S. Aime, A. Barge, M. Botta, D. Parker and A. S. De Sousa, J. Am. Chem. Soc., 1997, 119, 4767–4768 CrossRef CAS.
- C.-H. Huang, J. Hammell, S. J. Ratnakar, A. D. Sherry and J. R. Morrow, Inorg. Chem., 2010, 49, 5963–5970 CrossRef CAS PubMed.
- Sigmaplot for windows, Version 11, Dundas Software, Germany, 1999 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 949567 and 949568. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc48628a |
| ‡ DTMA = 1,4,7,10-tetrakis[(N-methylcarbamyl)methyl]-1,4,7,10-tetraazacyclododecane; CyD3MA = 1,4,7-tris[(N-methylcarbamyl)methyl]-10-(2-hydroxy-cyclohexyl)-1,4,7,10-tetraazacyclododecane. |
§ Crystallographic data for (CyD3MA·H2O): C23H45.56N7O4.28, M = 488.16, monoclinic, P21/c, a = 9.6189(2), b = 32.1984(7), c = 8.6632(2), α = 90, β = 99.5380(10)°, γ = 90, V = 2646.02(10) Å3, Z = 4, T = 173(2) K, R(int) 0.0288, final full-matrix least-squares refinement converged to R1 = 0.0763 (5204 reflections, F2, I > 2σ(I)); R1 = 0.0936 and wR2 = 0.1817 for all 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 reflections, 628 parameters, 1248 restraints, goodness-of-fit (S) 1.143; CCDC 949568. Crystallographic data for [Eu(CyD3MA)(CF3SO3)](CF3SO3)2(CH3CN)1.5: C29H49.5EuF9N8.5O13S3, M = 11444.41, triclinic, P 521 reflections, 628 parameters, 1248 restraints, goodness-of-fit (S) 1.143; CCDC 949568. Crystallographic data for [Eu(CyD3MA)(CF3SO3)](CF3SO3)2(CH3CN)1.5: C29H49.5EuF9N8.5O13S3, M = 11444.41, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 11.8523(2), b = 13.4938(2), c = 15.4748(3), α = 70.5800(10)°, β = 70.7790(10)°, γ = 81.9570(10)°, V = 2202.72(7) Å3, Z = 2, T = 173(2) K, R(int) 0.0338, final full-matrix least-squares refinement converged to R1 = 0.0234 (10631 reflections, F2, I > 2σ(I)); R1 = 0.0257 and wR2 = 0.0637 for all 30 , a = 11.8523(2), b = 13.4938(2), c = 15.4748(3), α = 70.5800(10)°, β = 70.7790(10)°, γ = 81.9570(10)°, V = 2202.72(7) Å3, Z = 2, T = 173(2) K, R(int) 0.0338, final full-matrix least-squares refinement converged to R1 = 0.0234 (10631 reflections, F2, I > 2σ(I)); R1 = 0.0257 and wR2 = 0.0637 for all 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492 reflections, 595 parameters, 0 restraints, goodness-of-fit (S) 1.037; CCDC 949567. 492 reflections, 595 parameters, 0 restraints, goodness-of-fit (S) 1.037; CCDC 949567. |
| ¶ Spectroscopic data was acquired using Cary Eclipse Fluorescence and Cary 300 Bio UV-vis spectrometers. Hellma quartz SUPARSIL cells, path length 10 mm, were used: type 111-QS for lifetime and phosphorescence data, type 110-QS for UV-vis data. Buffered solutions of [Eu(CyD3MA)](CF3SO3)3 (1.0 mM) were prepared in deionised water and pH adjustments performed using concentrated NaOH/NaOD and HCl/DCl. Deprotonation constants were established using Sigmaplot programme.21 Synthesis of all compounds in ESI.† |
| This journal is © The Royal Society of Chemistry 2014 |