Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High density monolayer of diisocyanide on gold surface as a platform of supported Rh-catalyst for selective 1,4-hydrogenation of α,β-unsaturated carbonyl compounds†
S.
Jagtap
a,
Y.
Kaji
b,
A.
Fukuoka
a and
K.
Hara
*a
aCatalysis Research Centre, Hokkaido University, Kita 21 Nishi 10, Kita-ku, Sapporo, Hokkaido 001-0021, Japan. E-mail: hara@cat.hokudai.ac.jp; Fax: +81 11 706 9139; Tel: +81 11 706 9136
bGraduate School of Science, Hokkaido University, Kita 10 Nishi 8, Kita-ku, Sapporo, Hokkaido 060-0810, Japan
First published on 4th March 2014
Abstract
A high density monolayer of diisocyanide on gold surface was utilized as a platform of supported Rh catalyst for selective 1,4-hydrogenation of α,β-unsaturated carbonyl compounds. The catalyst exhibited high turnover numbers in a range of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 150
000 to 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per Rh atom and showed steady catalyst performance over six recycle usages.
000 per Rh atom and showed steady catalyst performance over six recycle usages.
Self-assembling of organic molecules on surfaces automatically forms ordered and densely packed molecular monolayers. This spontaneous phenomenon can be used as a low-cost method to prepare functional materials for a wide range of applications including catalysts, sensors, photo- and electronic devices. In addition to the ease in preparation as mentioned above, another advantage of using self-assembled monolayers for catalyst preparation is its potential possibility to exhibit novel catalytic performance originated from its high density. For example, adjacent active centers on the top of a monolayer may synergistically enable unique catalytic activity and selectivity. However, such unique catalytic performance of high-density monolayers has been reported in a limited number of publications.1–3 Milstein et al. reported that monolayer and Langmuir–Blodgett films of a rhodium–bipyridine complex show high catalyst turnover numbers in hydrogenation of acetone as well as a unique selectivity towards acetone in the presence of butanone.1 We previously utilized a monolayer of alkanethiolate on gold surface4 to prepare a high-density monolayer of rhodium–phosphine complex for dehydrogenative silylation of alcohols and obtained complete selectivity towards primary alcohols over secondary alcohols.3 If more examples of advantageous features are demonstrated with high-density monolayer catalysts, their potential application in catalysis will be increasingly recognized. We thus envisioned the utilization of a monolayer of isocyanide for catalyst preparation. Compared to commonly used self-assembled monolayers of alkanethiolate on gold surfaces,4 monolayers of isocyanide have been much less studied. Although fundamental studies on formation of isocyanide monolayers5 as well as microscopic and spectroscopic studies6 have been reported, its application has not been extensively explored. Immobilization of ruthenium phthalocyanine on an isocyanide monolayer was reported;7 however, application of the metal-immobilized monolayer was not shown. In this study, a densely-packed monolayer of diisocyanide molecule on gold surface was utilized for high-density immobilization of a rhodium complex. The Rh-immobilized monolayer showed high catalyst turnover numbers in selective 1,4-hydrogenation of α,β-unsaturated carbonyl compounds as well as steady catalyst performance over at least six recycle usages.
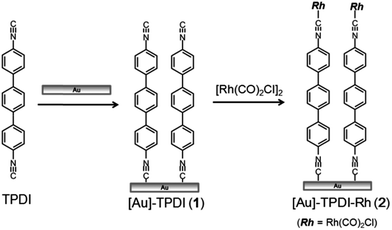
As illustrated in Fig. 1, immersion of a gold surface (evaporated on glass) in a 1.0 mM solution of 4,4′′-p-terphenyl diisocyanide (TPDI) in CH2Cl2 for 24 h, followed by washing with CH2Cl2, afforded [Au]–TPDI (1).5 Complexation of 1 with Rh to obtain [Au]–TPDI–Rh (2) was carried out by immersion of monolayer 1 into a 5.0 mM solution of [Rh(CO)2Cl]2 in benzene for 3 h, followed by washing with benzene.
IR-RAS measurement of [Au]–TPDI (1) revealed the stretching modes of both Au surface bound (2195 cm−1) and free (2122 cm−1) isocyanides as shown in Fig. 2a.5 In contrast, the IR-RAS spectrum of [Au]–TPDI–Rh (2) (Fig. 2b) showed no peak for the free isocyanide whereas a new peak at 2165 cm−1, which can be assigned to Rh-bound isocyanide, was observed. The peaks appearing at 2025 and 2091 cm−1 are at similar wavenumbers with those of a corresponding mixture of isocyanide and Rh precursor (Fig. 2c), suggesting two carbonyl ligands are bound to the Rh atom in cis-position in square-planer coordination. These results indicate that all the free isocyanide groups on top of the diisocyanide monolayer (1) were coordinated to Rh in the preparation of [Au]–TPDI–Rh (2). ICP-MS measurement of [Au]–TPDI–Rh (2) gave a Rh concentration of 0.66 nmol cm−2. Based on this surface concentration of Rh and the assumption of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation between Rh and isocyanide, a space-filling model of [Au]–TPDI–Rh (2) can be drawn as shown in Fig. S1 (ESI†). Considering the sizes of TPDI molecule and the Rh species, formation of the densely-packed monolayer in [Au]–TPDI (1) and complete Rh complexation in [Au]–TPDI–Rh (2) could be reasonable.
1 complex formation between Rh and isocyanide, a space-filling model of [Au]–TPDI–Rh (2) can be drawn as shown in Fig. S1 (ESI†). Considering the sizes of TPDI molecule and the Rh species, formation of the densely-packed monolayer in [Au]–TPDI (1) and complete Rh complexation in [Au]–TPDI–Rh (2) could be reasonable.
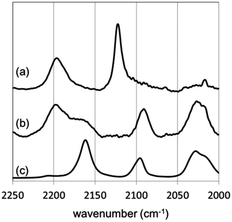 | ||
Fig. 2 IR-RAS spectra of (a) [Au]–TPDI (1) and (b) [Au]–TPDI–Rh (2), and (c) FT-IR spectrum of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4-biphenyl isocyanide (BPI) and [RhCl(CO)2]2 (isocyanide/Rh ratio = 1) in CH2Cl2. 1 mixture of 4-biphenyl isocyanide (BPI) and [RhCl(CO)2]2 (isocyanide/Rh ratio = 1) in CH2Cl2. | ||
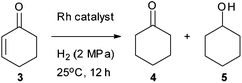
While abundant literature is available on the use of various homogeneous and heterogeneous catalysts for selective hydrogenation of α,β-unsaturated carbonyl compounds, there are still several problems associated with catalyst recyclability and lower catalyst turnover number.8 The catalytic activity of [Au]–TPDI–Rh (2) was thus examined in the hydrogenation of 2-cyclohexen-1-one (3) as a model substrate (Table 1). Accordingly, a single chip of [Au]–TPDI–Rh (2) (5 mm × 5 mm) was placed at the bottom of a high-pressure reactor containing an EtOH solution of 3 (12 μmol). Under these reaction conditions, the substrate/catalyst (Rh atom) ratio is 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The hydrogenation reaction was conducted under 2 MPa at 25 °C for 12 h to afford 1,4-hydrogenated product 4 in 77% yield with only 2% yield of further hydrogenated product 5, with no formation of 1,2-hydrogenated product (2-cyclohexen-1-ol), so corresponding to a 1,4-selectivity of 97% (entry 3). Control experiments with gold surface alone (entry 1) and [Au]–TPDI (1) (entry 2) gave very sluggish and no reaction, respectively, which implies that catalytically active sites in [Au]–TPDI–Rh (2) are the immobilized Rh species. The hydrogenation in the presence of [RhCl(CO)2]2 as a homogeneous catalyst resulted in a very low yield and low 1,4-selectivity (entry 4). Notably, the reaction with [RhCl(CO)2]2 after another 8 h gave no significant increase in yield (entry 5), showing severe deactivation of catalytically active species. Although another homogeneous control experiment using a 2
000. The hydrogenation reaction was conducted under 2 MPa at 25 °C for 12 h to afford 1,4-hydrogenated product 4 in 77% yield with only 2% yield of further hydrogenated product 5, with no formation of 1,2-hydrogenated product (2-cyclohexen-1-ol), so corresponding to a 1,4-selectivity of 97% (entry 3). Control experiments with gold surface alone (entry 1) and [Au]–TPDI (1) (entry 2) gave very sluggish and no reaction, respectively, which implies that catalytically active sites in [Au]–TPDI–Rh (2) are the immobilized Rh species. The hydrogenation in the presence of [RhCl(CO)2]2 as a homogeneous catalyst resulted in a very low yield and low 1,4-selectivity (entry 4). Notably, the reaction with [RhCl(CO)2]2 after another 8 h gave no significant increase in yield (entry 5), showing severe deactivation of catalytically active species. Although another homogeneous control experiment using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4-biphenyl isocyanide (BPI) and [RhCl(CO)2]2 facilitated the hydrogenation reaction, the 1,4-selectivity was <90% even in an initial stage of the reaction where 26% of 4 was formed (entry 6). These experiments indicate that Rh–diisocyanide monolayer 2 exhibits a uniquely high 1,4-selectivity. The catalyst turnover number obtained with [Au]–TPDI–Rh (2) was 55
1 mixture of 4-biphenyl isocyanide (BPI) and [RhCl(CO)2]2 facilitated the hydrogenation reaction, the 1,4-selectivity was <90% even in an initial stage of the reaction where 26% of 4 was formed (entry 6). These experiments indicate that Rh–diisocyanide monolayer 2 exhibits a uniquely high 1,4-selectivity. The catalyst turnover number obtained with [Au]–TPDI–Rh (2) was 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in 12 h. Increase in the reaction scale to 30 μmol of 3 afforded a catalyst turnover number of 147
000 in 12 h. Increase in the reaction scale to 30 μmol of 3 afforded a catalyst turnover number of 147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with 82% yield of 4 and 98% 1,4-selectivity in 12 h. This experiment demonstrates the feasibility of scale-up of the present catalyst system. For practical purposes, chemical engineering methodologies, such as microfluidic systems, may further enhance the efficiency of the present catalytic system.
000 with 82% yield of 4 and 98% 1,4-selectivity in 12 h. This experiment demonstrates the feasibility of scale-up of the present catalyst system. For practical purposes, chemical engineering methodologies, such as microfluidic systems, may further enhance the efficiency of the present catalytic system.
| Entry | Catalyst | Yield 4 (%) | Yield 5 (%) | 1,4-Selectivity (%) |
|---|---|---|---|---|
a
Reaction conditions: 2-cyclohexene-1-one 12 μmol, 2-cyclohexene-1-one/Rh = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, EtOH 120 μL, H2 2 MPa, 25 °C, 12 h.
b Reaction was carried out for 20 h.
c Reaction was carried out for 2 h. 2 000, EtOH 120 μL, H2 2 MPa, 25 °C, 12 h.
b Reaction was carried out for 20 h.
c Reaction was carried out for 2 h. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4-biphenyl isocyanide (BPI) and [RhCl(CO)2]2 was used as catalyst (isocyanide/Rh ratio = 1). 1 mixture of 4-biphenyl isocyanide (BPI) and [RhCl(CO)2]2 was used as catalyst (isocyanide/Rh ratio = 1).
|
||||
| 1 | Au surface | 5 | 2 | 71 |
| 2 | [Au]–TPDI (1) | 0 | 0 | — |
| 3 | [Au]–TPDI–Rh (2) | 77 | 2 | 97 |
| 4 | [RhCl(CO)2]2 | 8 | 15 | 35 |
| 5b | [RhCl(CO)2]2 | 10 | 19 | 34 |
| 6c | BPI–RhCl(CO)2 | 26 | 4 | 87 |
Choice of the solvent was found to be particularly important since it determines the activity and the stability of catalyst [Au]–TPDI–Rh (2). The reactions in n-hexane9 and toluene resulted in lower turnover numbers of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 19
000 and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. The use of isopropanol gave further sluggish reaction, indicating that a hydrogen transfer step is less likely involved in the reaction mechanism. Although the use of THF afforded a relatively high turnover of 66
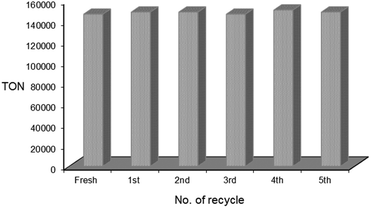
000, respectively. The use of isopropanol gave further sluggish reaction, indicating that a hydrogen transfer step is less likely involved in the reaction mechanism. Although the use of THF afforded a relatively high turnover of 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the catalyst activity of 2 completely disappeared after the second recycle run. In contrast, the catalytic performance of 2 in EtOH was maintained during repeated uses without any significant loss of activity even after the fifth recycle run (Fig. 3). The total catalyst turnover number of 2 in EtOH over the six successive runs reached 892
000, the catalyst activity of 2 completely disappeared after the second recycle run. In contrast, the catalytic performance of 2 in EtOH was maintained during repeated uses without any significant loss of activity even after the fifth recycle run (Fig. 3). The total catalyst turnover number of 2 in EtOH over the six successive runs reached 892![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
Application of [Au]–TPDI–Rh (2) for the hydrogenation of various α,β-unsaturated compounds is summarized in Table 2. α,β-Unsaturated ketones and esters were selectively converted into the corresponding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenated products in high yields (entries 1–6). Citral can also be converted to 1,4-hydrogenated product, citronellal, in 65% yield with 98% 1,4-selectivity (entry 7).
C hydrogenated products in high yields (entries 1–6). Citral can also be converted to 1,4-hydrogenated product, citronellal, in 65% yield with 98% 1,4-selectivity (entry 7).
The high selectivities obtained by using [Au]–TPDI–Rh (2) might be associated with preferred adsorption and/or coordination interaction of the starting enones with the catalyst surface over that of the ketones. More detailed experiments to clarify the origin of the selectivities are now under investigation in our lab.
In summary, a monolayer of diisocyanide on gold surface was utilized to immobilize catalytically active Rh species on the top of the monolayer. IR-RAS and ICP-MS analyses of the Rh-immobilized surface confirmed the formation of a high-density monolayer of Rh–diisocyanide. The Rh-immobilized monolayer exhibited extremely high catalyst turnover numbers and uniquely high 1,4-selectivity for hydrogenation reaction of various α,β-unsaturated carbonyl compounds with high recyclability. It can be noted that the catalyst preparation in the present approach using high-density monolayer of metal complexes has unique features against the conventional heterogeneous and homogeneous catalyst systems. Further applications based on the present approach by using simple wet processes are expected to show its versatile significance in various catalytic reactions.
Prof. T. Kawaguchi and Prof. K. Shimazu are gratefully acknowledged for preparation of gold surfaces, Prof. T. Watanabe is acknowledged for ICP-MS measurement and Mr K. Namba for experimental help. This work was financially supported by the Asahi Glass Foundation, JSPS KAKENHI Grant Numbers 23105503, 25105703 and the Global COE Program (Project No. B01: Catalysis as the Basis for Innovation in Materials Science) from MEXT, Japan.
Notes and references
- K. Töllner, R. Popovitz-Biro, M. Lahav and D. Milstein, Science, 1997, 278, 2100 CrossRef
.
- I. O. Benítez, B. Bujoli, L. J. Camus, C. M. Lee, F. Odobel and D. R. Talham, J. Am. Chem. Soc., 2002, 124, 4363 CrossRef CAS PubMed
; A. Pasc-Banu, C. Sugisaki, T. Gharsa, J.-D. Marty, I. Gascon, G. Pozzi, S. Quici, I. Rico-Lattes and C. Mingotaud, Angew. Chem., Int. Ed., 2004, 43, 6174 CrossRef PubMed
; M. Bartz, J. Küther, R. Seshadri and W. Tremel, Angew. Chem., Int. Ed., 1998, 37, 2466 CrossRef
; K. Marubayashi, S. Takizawa, T. Kawakusu, T. Arai and H. Sasai, Org. Lett., 2003, 5, 4409 CrossRef PubMed
.
- K. Hara, R. Akiyama, S. Takakusagi, K. Uosaki, T. Yoshino, H. Kagi and M. Sawamura, Angew. Chem., Int. Ed., 2008, 47, 5627 CrossRef CAS PubMed
.
- A. Ulman, Chem. Rev., 1996, 96, 1533 CrossRef CAS PubMed
; A. Ulman, Thin Films: Self-Assembled Monolayers of Thiols, Academic Press, San Diego, CA, 1998 CrossRef
; S. Flink, F. C. J. M. van Veggel and D. N. Reinhoudt, Adv. Mater., 2000, 12, 1315 CrossRef
; J. J. Gooding, F. Mearns, W. Yang and J. Liu, Electroanalysis, 2003, 15, 81 CrossRef PubMed
; J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef PubMed
.
- J. I. Henderson, S. Feng, T. Bein and C. P. Kubiak, Langmuir, 2000, 16, 6183 CrossRef CAS
; S. A. Swanson, R. McClain, K. S. Lovejoy, N. B. Alamdari, J. S. Hamilton and J. C. Scott, Langmuir, 2005, 21, 5034 CrossRef PubMed
.
- J. A. Boscoboinik, F. C. Calaza, Z. Habeeb, D. W. Bennett, D. J. Stacchiola, M. A. Purino and W. T. Tysoe, Phys. Chem. Chem. Phys., 2010, 12, 11624 RSC
; J. Boscoboinik, J. Kestell, M. Garvey, M. Weinert and W. T. Tysoe, Top. Catal., 2011, 54, 20 CrossRef CAS
; K. Ikeda, N. Fujimoto, H. Uehara and K. Uosaki, Chem. Phys. Lett., 2008, 460, 205 CrossRef PubMed
; K. Ikeda, J. Sato, N. Fujimoto, N. Hayazawa, S. Kawata and K. Uosaki, J. Phys. Chem. C, 2009, 113, 11816 Search PubMed
; M. Ito, H. Noguchi, K. Ikeda and K. Uosaki, Phys. Chem. Chem. Phys., 2010, 12, 3156 RSC
.
- V. Huc, J.-P. Bourgoin, C. Bureau, F. Valin, G. Zalczer and S. Palacin, J. Phys. Chem. B, 1999, 103, 10489 CrossRef CAS
.
- F. H. Jardine and G. Wilkinson, J. Chem. Soc. C, 1967, 270 RSC
; J. Tsuji and K. Ohno, Tetrahedron Lett., 1967, 8, 2173 CrossRef CAS
; J. Tsuji and K. Ohno, Synthesis, 1969, 157 CrossRef
; V. V. Grushin and H. Alper, Organometallics, 1991, 10, 831 CrossRef
.
- Preliminary XPS results showed the formation of Rh(0) species by a hydrogenation reaction in hexane, which is most probably the reason for lower catalyst turnover number.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures for catalyst preparation and characterization; space-filling model of catalyst. See DOI: 10.1039/c3cc48181f |
| This journal is © The Royal Society of Chemistry 2014 |