SERS-based competitive immunoassay of troponin I and CK-MB markers for early diagnosis of acute myocardial infarction†
Hyangah
Chon
a,
Sangyeop
Lee
a,
Soo-Young
Yoon
b,
Eun Kyu
Lee
c,
Soo-Ik
Chang
d and
Jaebum
Choo
*a
aDepartment of Bionano Engineering, Hanyang University, Ansan 426-791, South Korea. E-mail: jbchoo@hanyang.ac.kr
bDepartment of Laboratory Medicine, Korea University College of Medicine, Ansan 425-707, South Korea
cCollege of Bionanotechnology, Gachon University, Sungnam 461-701, South Korea
dDepartment of Biochemistry, Chungbuk National University, Cheongju 361-763, South Korea
First published on 18th November 2013
Abstract
We report a SERS-based competitive immunoassay technique for the early diagnosis of acute myocardial infarction (AMI). Simultaneous quantification of the dual cardiac markers, CK-MB and troponin I, was achieved by single wavelength excitation.
Modern trends in the early diagnosis of acute myocardial infarction (AMI) involve the accurate determination of serum levels of a group of cardiac biomarkers.1 Among the most frequently used biomarkers, isoenzyme MB of creatine kinase (CK-MB)2 and troponin I (cTnI)3 have been demonstrated clinically to be the most sensitive and selective cardiac markers. Therefore, simultaneous determination of CK-MB and cTnI in blood provides many advantages over separate determination of each marker because it dramatically increases the probability of an early diagnosis of AMI. Detection time is another important factor for early diagnosis of AMI.4 According to the US National Heart Attack Alert Program report, the benefit of treatment to patients with suspected AMI is decreased tremendously if therapy is delayed for more than 2 h.5 Thus, the development of a rapid and simultaneous detection technique for cTnI and CK-MB in blood is absolutely necessary for early diagnosis of AMI.
Several immunoassay technologies, including fluorescence detection,6 chemiluminescence,7 electrochemical detection8 and surface plasmon resonance,9 have been employed for the simultaneous quantification of cTnI and CK-MB in patient serum. However, these detection methods suffer from poor sensitivity, low precision and long assay time. Recently, a surface-enhanced Raman scattering (SERS)-based immunoassay technique using functional nanoparticles has attracted significant attention due to its high sensitivity and potential for multiplexing.10 In particular, SERS has been considered as a potential candidate for multiplex detection because Raman peaks are 100 times narrower than fluorescence bands.11 One of the recently developed SERS assay platforms is a sandwich immunoassay using SERS nano-tags and magnetic beads.12 This method employs two antibodies that bind to different sites on an antigen. Specifically, the primary antibody is attached to the surface of magnetic beads, to which antigens and a secondary antibody conjugated with a SERS nano-tag are added sequentially. As a result, the antigen is ‘sandwiched’ between the two antibodies. The binding affinity of the antibody to the antigen is the main determinant of immunoassay sensitivity. However, this SERS-based sandwich immunoassay suffers from a long assay time and cross reactivity.
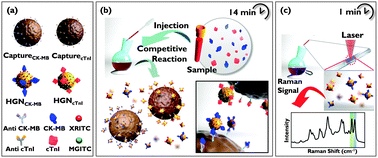
To address these problems, we developed a SERS-based competitive immunoassay. Fig. 1 illustrates the competitive assay process for the simultaneous detection of cTnI and CK-MB. First, monoclonal antibodies for cTnI and CK-MB were immobilized onto magnetic beads while target antigens (cTnI and CK-MB) were conjugated onto the surface of two different types of SERS nano-tags. When free target antigens and the antigens conjugated onto the surface of SERS nano-tags were mixed with magnetic beads, they reacted competitively with the monoclonal antibodies on the magnetic beads. The immunocomplexes were then separated using a small magnetic bar, and the Raman signals of the remaining SERS nano-tags in the supernatant were measured. Using the SERS-based competitive assay technique, simultaneous detection of cTnI and CK-MB cardiac markers in blood serum could be successfully achieved at a single excitation wavelength. This competitive assay required no sample processing, beyond centrifugation or filtration to remove particulates, and was significantly less sensitive to sample dilution and matrix effects than the sandwich immunoassay format. Therefore, the method described here provided much reproducible data with lower variability between duplicate samples. To the best of our knowledge, this is the first report about a SERS-based competitive immunoassay for the simultaneous detection of two cardiac markers. We anticipate that this new SERS-based diagnostic technique satisfies the current need for early diagnosis of AMI.
For simultaneous detection of dual biomarkers, two different types of SERS nano-tags were prepared. Specifically, malachite green isothiocyanate (MGITC) and X-rhodamine-5-(and-6)-isothiocyanate (XRITC) were used as Raman reporter molecules, respectively. After adsorption of Raman reporter molecules, cTnI and CK-MB antigens were conjugated to the surface of hollow gold nanospheres (HGNs). The advantages of HGNs as SERS probes have been reported elsewhere.13 To confirm the quantitative analytical capabilities of HGNMGITC-cTnI and HGNXRITC-CK-MB SERS nano-tags, the SERS spectra for different molar ratios were recorded and analyzed (Fig. S1 in ESI†). The change in SERS intensity after mixing the SERS nano-tags with capture beads was monitored as a function of time to identify the optimal reaction time for the immunoreactions of cTnI and CK-MB. Competitive immunoreactions were almost completed after seven minutes for both antigens (Fig. S2 in ESI†), and thus the optimal reaction time between SERS nano-tags and capture beads was determined to be seven minutes.
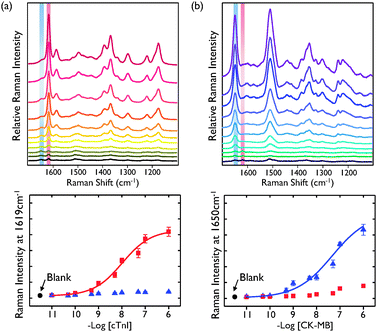
Minimum cross-reactivity or interference among different sets of antigens and antibodies is an essential criterion to achieve good selectivity in multiplexed immunoassays. Thus, the cross reactivity between cTnI and CK-MB was evaluated for a concentration range of 10 pg mL−1 to 1 μg mL−1. Fig. 2a shows the SERS spectra for nine different concentrations of cTnI and their corresponding calibration curves. The relative Raman intensity of cTnI (red square) at 1619 cm−1 was monitored and used as a quantitative evaluation of cTnI antigen levels. As shown in the calibration curve, the SERS intensity increased with increasing concentrations of cTnI. In this case, however, there was no change in SERS intensity for CK-MB (blue triangle) at 1650 cm−1. On the other hand, the SERS intensity for CK-MB increased with increasing CK-MB concentration, while the SERS intensity at 1650 cm−1 for cTnI was not changed (Fig. 2b). Together, these results indicated that there was no serious cross-reactivity in the SERS-based competitive assay.
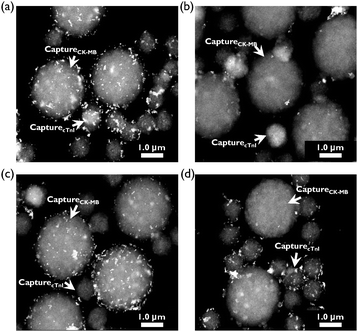
To confirm the potential applicability of the simultaneous duplex assay, two different sizes of monoclonal antibody-conjugated magnetic beads (1 μm diameter: CapturecTnI and 3 μm diameter: CaptureCK-MB) were prepared and used as supporting substrates. Fig. 3 shows TEM images of two different sizes of magnetic beads (CapturecTnI and CaptureCK-MB) for four different sample conditions. When no cTnI and CK-MB markers were present in the solution, HGNMGITC-cTnI and HGNXRITC-CK-MB SERS nano-tags were freely bound to CapturecTnI and CaptureCK-MB, respectively (Fig. 3a). On the other hand, SERS nano-tags could not bind to the surface of magnetic beads at concentrations less than 100 ng mL−1 for cTnI and 100 ng mL−1 for CK-MB because both capture beads were blocked by free cTnI and CK-MB antigens as shown in Fig. 3b. At concentrations of less than 100 ng mL−1 of cTnI only, HGNXRITC-CK-MB SERS nano-tags were bound to the large-sized capture beads (Fig. 3c), while HGNMGITC-cTnI SERS nano-tags were bound to the small-sized capture beads in the opposite situation (Fig. 3d). Taken together, the TEM images illustrate that immunocomplexes between SERS nano-tags and the capture beads did not form if both antigens were present in the solution, but did form in the absence of antigen. In addition, the images also showed their selective interactions along the concentration variations of cTnI and CK-MB.
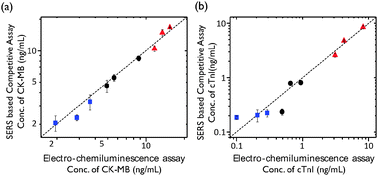
We next evaluated the clinical application of our SERS-based competitive immunoassay. To assess the analytical reliability and clinical application potential of our proposed method, we compared it to commercially measured assay data. For experiments, nine different blood samples for each target marker (a total of eighteen samples) were collected from patients at the Korea University Medical Center. According to the manufacturer's protocol, specimens with a CK-MB value of 4.87 ng mL−1 and a cTnI value of 0.30 ng mL−1 were considered pathologically positive. The SERS-based competitive assay for dual markers was performed in parallel. Before performing the immunoassay on human serum using SERS nano-tags, a calibration curve for CK-MB and cTnI was generated using known concentrations of CK-MB and cTnI prepared in a solution of FBS. The SERS intensities of CK-MB and cTnI from the sera of nine different patients at 1619 cm−1 and 1650 cm−1 were measured, and the amounts of CK-MB and cTnI were quantitatively determined based on the calibration curve. In addition, the reliability of the assays developed was evaluated by assaying eighteen human serum samples (nine for cTnI and nine for CK-MB) using a SERS-based immunosensor and commercially available electroluminescent micro-particle assay kit (Roche Cobas) for cTnI and CK-MB. As shown in Fig. 4, the values determined using the proposed SERS-based immunosensor were in good agreement with those obtained using the electro-chemiluminescent assay kit for CK-MB (Fig. 4a) and cTnI (Fig. 4b).
The limits of detection (LODs) for CK-MB and cTnI were determined to be 42.5 pg mL−1 and 33.7 pg mL−1 from three standard deviations above the background, respectively. Consequently, the SERS-based competitive immunoassay technique was determined to be approximately 100 to 1000 times more sensitive than that of the electro-chemiluminescent assay. To confirm the clinical applicability of our SERS-based competitive immunoassay for CK-MB and cTnI, Bland–Altman analysis14 (Fig. S5 in ESI†) and Passing–Bablok regression analysis15 (Fig. S6 in ESI†) have been performed using the commercially available ‘MedCalc’ program. As shown in Fig. S5a and S6a (ESI†), all the difference values between the SERS-based assay and the reference (Roche Cobas) assay were in 95% limit of agreement ranges in the Bland–Altman analysis. As shown in Fig. S5b and S6b (ESI†), the fitted regression line was within the acceptable 95% confidence interval range in the Passing–Bablok regression analysis. Thus, it can be concluded that all the values are in the clinically acceptable ranges, and the assay data determined by the SERS-based competitive immunoassay method are clinically valid.
In summary, we developed a fast and sensitive SERS-based competitive immunoassay technique using SERS nano-tags and magnetic capture beads. In this novel assay technique, free target antigens and antigen-conjugated HGN SERS nano-tags reacted competitively with monoclonal antibodies on magnetic capture beads. The simultaneous quantification of dual AMI biomarkers in patient serum, namely, cTnI and CK-MB, was successfully achieved at a single excitation wavelength. This SERS-based competitive immunoassay has multiple advantages including a quick assay time (less than 15 min), an easy assay procedure (using magnetic beads), small sample consumption (minimum of 10 μL) and simultaneous dual marker detection using reliable, sharp, and easily distinguishable SERS peaks. We anticipate that this SERS-based competitive immunoassay may open up new diagnostic methods for early AMI diagnosis.
The National Research Foundation of Korea supported this work through grant numbers R11-2008-0061852, 2012035286, and K20904000004-13A0500-00410.
Notes and references
- A. Dolci and M. Panteghini, Clin. Chim. Acta, 2006, 369, 179–187 CrossRef CAS PubMed.
- D. J. Robinson and R. H. Christenson, J. Emerg. Med., 1999, 17, 95–104 CrossRef CAS.
- J. Sarko and C. V. Pollack, J. Emerg. Med., 2002, 23, 57–65 CrossRef.
- N. Jordanova, M. Gyongyosi, A. Khorsand, C. Falkensammer, G. Zorn, J. Wojta, A. Anvari and K. Huber, Int. J. Cardiol., 2005, 99, 429–435 CrossRef PubMed.
- F. S. Apple, R. L. Jesse, K. Newby, A. H. B. Wu and R. H. Christenson, Circulation, 2007, 115, e352–e355 CrossRef PubMed.
- (a) M. M. Caulum, B. M. Murphy, L. M. Ramsey and C. S. Henry, Anal. Chem., 2007, 79, 5249–5256 CrossRef CAS PubMed; (b) L. Gervais and E. Delamarche, Lab Chip, 2009, 9, 3330–3337 RSC.
- (a) I. H. Cho, E. H. Paek, Y. K. Kim, J. H. Kim and S. H. Paek, Anal. Chim. Acta, 2009, 632, 247–255 CrossRef CAS PubMed; (b) F. Darain, P. Yager, K. L. Gan and S. C. Tjin, Biosens. Bioelectron., 2009, 24, 1744–1750 CrossRef CAS PubMed.
- (a) J. H. Chua, R. E. Chee, A. Agarwal, S. M. Wong and G. J. Zhang, Anal. Chem., 2009, 81, 6266–6271 CrossRef CAS PubMed; (b) D. Purvis, O. Leonardova, D. Farmakovsky and V. Cherkasov, Biosens. Bioelectron., 2003, 18, 1385–1390 CrossRef CAS.
- (a) J. F. Masson, L. Obando, S. Beaudoin and K. Booksh, Talanta, 2004, 62, 865–870 CrossRef CAS PubMed; (b) R. Kurita, Y. Yokota, Y. Sato, F. Mizutani and O. Niwa, Anal. Chem., 2006, 78, 5525–5531 CrossRef CAS PubMed.
- (a) S. A. Chen, Y. X. Yuan, J. L. Yao, S. Y. Han and R. A. Gu, Chem. Commun., 2011, 47, 4225–4227 RSC; (b) H. Zhang, M. H. Harpster, H. J. Park and P. A. Johnson, Anal. Chem., 2011, 83, 254–260 CrossRef CAS PubMed; (c) Y. Q. Wang, B. Yan and L. X. Chen, Chem. Rev., 2013, 113, 1391–1428 CrossRef CAS PubMed.
- (a) H. Chon, S. Lee, S. Y. Yoon, S.-I. Chang, D. W. Lim and J. Choo, Chem. Commun., 2011, 47, 12515–12517 RSC; (b) J. Huang, K. H. Kim, N. Choi, H. Chon, S. Lee and J. Choo, Langmuir, 2011, 27, 10228–10233 CrossRef CAS PubMed; (c) Z. Zhang, Y. Wen, Y. Ma, J. Luo, L. Jiang and Y. Song, Chem. Commun., 2011, 47, 7407–7409 RSC.
- (a) H. Chon, C. Lim, S. M. Ha, Y. Ahn, E. K. Lee, S.-I. Chang, G. H. Seong and J. Choo, Anal. Chem., 2010, 82, 5290–5295 CrossRef CAS PubMed; (b) M. Lee, S. Lee, J.-H. Lee, H.-W. Lim, G. H. Seong, E. K. Lee, S.-I. Chang, C. H. Oh and J. Choo, Biosens. Bioelectron., 2011, 26, 2135–2141 CrossRef CAS PubMed.
- (a) A. M. Schwartzberg, T. Y. Oshiro, J. Z. Zhang, T. Huser and C. E. Talley, Anal. Chem., 2006, 78, 4732–4736 CrossRef CAS PubMed; (b) H. Chon, S. Lee, S. W. Son, C. H. Oh and J. Choo, Anal. Chem., 2009, 81, 3029–3034 CrossRef CAS PubMed.
- J. M. Bland and D. G. Altman, Lancet, 1986, 1, 307–310 CrossRef CAS.
- H. Passing and W. Bablok, J. Clin. Chem. Clin. Biochem., 1983, 21, 709–720 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure. See DOI: 10.1039/c3cc47850e |
| This journal is © The Royal Society of Chemistry 2014 |