Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amide bond formation via C(sp3)–H bond functionalization and CO insertion†‡
Huizhen
Liu
,
Gabor
Laurenczy
,
Ning
Yan
and
Paul J.
Dyson
*
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland. E-mail: Paul.Dyson@epfl.ch; Fax: +41-21-6939865
First published on 30th October 2013
Abstract
An efficient method for the synthesis of amides via Pd-catalyzed oxidative carbonylation of C(sp3)–H bonds with CO and amines is described. The route efficiently provides substituted phenyl amides from alkanes.
The importance of amides in chemistry and biology is well recognized and, consequently, a variety of methods have been developed for their synthesis,1 with notable examples including the Schmidt, Schotten–Baumann and Ugi reactions.2 These methods are based on the reactions of activated acid derivatives (acid chlorides and anhydrides) or acid/base induced rearrangement reactions. Recently, attention has been devoted to developing new routes to amides that do not require acid or base, but to achieve this goal, relatively expensive starting materials such as aldehydes are required. Metal catalyzed routes enable amides to be generated from starting materials other than carboxylic acids and these reactions are summarized in two excellent review articles.3,4 Perhaps the most notable achievement in this regard has been the direct catalytic conversion of alcohols and amines into amides.5
The synthesis of amides involving transition metal catalyzed C–X (X = H, Br, I etc.) bond functionalization followed by carbonylation with CO has received considerable attention as it has a high atom economy.6 Seminal research includes the catalytic aminocarbonylation of alk-1-ynes7 and the application of a homogeneous PdCl2–PPh3 catalytic system for direct oxidative aminocarbonylation using CO and oxygen under basic conditions.8 The catalytic aminocarbonylation of alkenes using a Co/C catalyst has also been reported,9 and palladium catalyzed carbonylation reactions of aryl bromides have been used to prepare benzamides from aryl bromides at atmospheric pressure.10 Other notable developments include transition metal-free alkoxycarbonylation of aryl halides,11 and the synthesis of amides by the activation of aromatic C–H bonds.12 Rhodium13 and ruthenium14 complexes that catalyze the formation of amides by activating C(sp2)–H bonds have also been reported.
Palladium–phosphine complexes are widely used to catalyze the formation of carbon–carbon, carbon–nitrogen and carbon–oxygen bonds.15 Bis-phosphine ligands are particularly useful in these reactions and the influence of the ligand bite-angle on C–C and C–X bond forming cross coupling reactions has been reviewed.16 The wide bite-angle bis-phosphine, Xantphos, has found a number of important uses. Notably, Huang and co-workers reported the synthesis of esters from alkanes using a PdCl2–Xantphos catalyst in the presence of tBuOOtBu.17 Azidocarbonylation reactions may also be catalyzed by a Pd2(dba)3–Xantphos system18 and intermolecular amidation of aryl halides using Pd(OAc)2–Xantphos has also been reported.19 Pd-catalyzed direct oxidative carbonylation of allylic C–H bonds with carbon monoxide has also been reported.20 We found that PdCl2 combined with various bis-phosphines including Xantphos, Nixantphos, (±)-Binapo or (R)-Phanephos catalyze the formation of amides in the absence of acid or base, by the direct functionalization of C(sp3)–H bonds with subsequent CO insertion – the outcome of these studies is described herein.
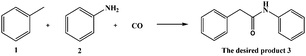
The viability of the reaction was explored with toluene 1 and aniline 2 as substrates under CO (50 atm) using various PdCl2-based catalysts due to their excellent performance in carbonylation reactions (Scheme 1, Table 1).21 High yields of the desired product (compound 3 in Scheme 1) are obtained with the wide bite-angle bis-phosphines, Xantphos, Nixantphos, or (R)-Phanephos and also with (±)-Binapo (Table 1, entries 9–12 and 16–19). The yield of 3 is very low in the absence of a ligand co-catalyst (Table 1, entries 1 and 2) or in the presence of other bis-phosphines and mono-phosphine ligands (Table 1, entries 3–8). The influence of the bis-phosphine bite-angle is apparent (Table 1, entries 3–12), with ligands with bite-angles >90° generally exhibiting better activity (the exception being dppf). Moreover, in the absence of an oxidant or in the presence of a weak oxidant, i.e. Ag2O, compound 3 is not obtained (Table 1, entries 21 and 22). Aniline reacts more favourably with itself to afford 1,3-diphenylurea when H2O2 is employed as the oxidant or H2O as the solvent (Table 1, entries 24 and 26). The influence of temperature and CO pressure on the carbonylation reaction was also investigated under the reaction conditions optimized using the PdCl2–Xantphos system. The highest yield of 66% is obtained when the reaction is performed under 50 atm of CO at 125 °C (Table 1, entry 16). Under these conditions a similar yield (68%) is obtained with the (R)-Phanephos ligand (Table 1, entry 19). Pd(0) pre-catalysts were also evaluated, i.e. Pd2(dba)3 and Pd(PPh3)4, and while they are active the desired product is obtained in low yield, 3% and 0.4%, respectively (Table 1, entries 27 and 28).
| Entry | T (°C) | Ligand | Bite angle, β° | Oxidant | Yield of 3g (%) |
|---|---|---|---|---|---|
| Dppbe = 1,2-bis(diphenylphosphino)benzene, Tapp = tris(o-methoxyphenyl)phosphine, Dppf = 1,1′-bis(diphenylphosphino)ferrocene, Dppe = 1,2-bis(diphenylphosphino)ethane, DTBP = tBuOOtBu. Reaction conditions: 1 (15 ml), 2 (1 mmol), PdCl2 (5 mol% based on aniline), ligand (0.06 mmol), oxidant (1.2 mmol), CO (50 atm), 24 h.a Without PdCl2.b CO (30 atm).c CO (40 atm).d With H2O (15 ml), 1 (15 mmol), and 2 (1 mmol).e Pd2(dba)3 0.05 mmol.f Pd(PPh3)4 0.05 mmol.g Yields were determined by GC analysis relative to aniline with n-decane as an internal standard.h The bite angle given corresponds to that in (Dppbe)PdBr2.i The structure of Nixantphos is similar to Xantphos and it is therefore assumed that their bite-angles are the same. | |||||
| 1 | 100 | — | — | DTBP | 0.4 |
| 2a | 100 | Xantphos | — | DTBP | 0 |
| 3 | 100 | Dppbe | 8722![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
DTBP | 0.9 |
| 4 | 100 | Triphos | 8423 | DTBP | 2 |
| 5 | 100 | PPh3 | — | DTBP | 10 |
| 6 | 100 | Dppe | 8624 | DTBP | 0 |
| 7 | 100 | Tapp | — | DTBP | 16 |
| 8 | 100 | Dppf | 9924 | DTBP | 3 |
| 9 | 100 | Xantphos | 10224 | DTBP | 34 |
| 10 | 100 | Nixantphos | 102i | DTBP | 36 |
| 11 | 100 | (±)-Binapo | 9325 | DTBP | 31 |
| 12 | 100 | (R)-Phanephos | 10126 | DTBP | 46 |
| 13b | 100 | Xantphos | 10224 | DTBP | 21 |
| 14c | 100 | Xantphos | 10224 | DTBP | 30 |
| 15 | 80 | Xantphos | 10224 | DTBP | 20 |
| 16 | 125 | Xantphos | 10224 | DTBP | 66 |
| 17 | 125 | Nixantphos | 102g | DTBP | 54 |
| 18 | 125 | (±)-Binapo | 9325 | DTBP | 62 |
| 19 | 125 | (R)-Phanephos | 10126 | DTBP | 68 |
| 20 | 150 | Xantphos | 10224 | DTBP | 48 |
| 21 | 125 | Xantphos | 10224 | — | 0 |
| 22 | 125 | Xantphos | 10224 | Ag2O | 0 |
| 23 | 125 | Xantphos | 10224 | AgOAc | 0 |
| 24 | 125 | Xantphos | 10224 | H2O2 | 0.3 |
| 25 | 125 | Xantphos | 10224 | K2S2O8 | 7 |
| 26d | 125 | Xantphos | 10224 | DTBP | 0 |
| 27e | 125 | Xantphos | 10224 | DTBP | 3 |
| 28f | 125 | Xantphos | 10224 | DTBP | 0.4 |
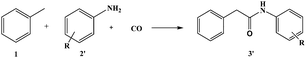
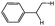
Under standard conditions toluene was replaced by toluene-d8 and after 2 hours the yield of the desired product is 7% (compared to 18% for toluene – rH/rD = 2.6) suggesting that the C(sp3)–H bond cleavage step occurs before the rate-limiting step or might be involved in the rate-limiting step of this transformation. Hence, the yield of the desired product should be related to the C(sp3)–H bond dissociation energies. The substrate scope of the reaction was explored under optimized conditions (CO 50 atm, 125 °C) using 5 mol% of PdCl2 with Xantphos or (R)-Phanephos and DTBP as the oxidant (Table 2). Products resulting from carbonylation of C(sp2)–H (aromatic) bonds are not observed (Table 2, entry 1). Cyclohexane reacts in the presence of DTBP to afford the corresponding amide in reasonable yield (Table 2, entries 4 and 5), albeit lower than the yield of the product obtained with toluene (Table 2, entries 6 and 7), presumably due to the higher bond dissociation energy of the C–H bond in cyclohexane compared to toluene.27 With diphenylmethane the main product is 1,3-diphenylurea, presumably due to steric hindrance. Presumably the yield of the branched product is higher than the linear product using ethylbenzene as the substrate for the same reason (Table 2, entries 2 and 3). Moreover, electron withdrawing –F and –Cl substituents in the para-position favor the reaction; however, for the carbonylation of toluene, ethylbenzene and cyclohexane, the ligand (R)-Phanephos is superior to Xantphos, whereas for substrates with electron withdrawing –F and –Cl substituents in the para-position, Xanthphos is superior (Table 2, entries 2–11). These combined data confirm that the yield of the desired product is related, at least in part, to the C(sp3)–H bond dissociation energies. The PdCl2–Xantphos catalyst tolerates anilines with electron withdrawing or donating substituents (Scheme 2 and Table 3), although electron withdrawing groups are more favorable for this reaction. The system is, unfortunately, inactive with alkylamines.
| Entry | RH | Yielda (%) |
|---|---|---|
| Reaction conditions: RH (15 ml), 2 (1 mmol), PdCl2 (5 mol% based on aniline), Xantphos (0.06 mmol), DTBP (1.2 mmol), CO (50 atm), 125 °C, 24 h.a Yields were determined by GC analysis relative to aniline with n-decane as an internal standard (isolated yield in parentheses).b With (R)-Phanephos (0.06 mmol).c N,2-diphenylpropanamide.d N,3-diphenylpropanamide. | ||
| 1 |

|
0 |
| 2 |
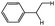
|
Branched (46)c Linear (13)d |
| 3b |
|
Branched (53)c Linear (12)d |
| 4 |
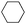
|
43 (39) |
| 5b |
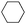
|
60 |
| 6 |
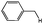
|
66 (62) |
| 7b |
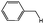
|
68 |
| 8 |
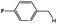
|
55 (51), 38b |
| 10 |
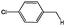
|
57 (51), 31b |
| 12 |
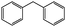
|
Trace |
| Entry | R | Yield of 3′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
|---|---|---|
| Reaction conditions: 1 (15 ml), 2′ (1 mmol), PdCl2 (5 mol% based on 2′), Xantphos (1.20 mmol) DTBP (1.2 mmol), CO (50 atm), 125 °C, 24 h.a Isolated yield based on the amine. | ||
| 1 | H | 62 |
| 2 | 3-NO2 | 64 |
| 3 | 3-CN | 57 |
| 4 | 4-CN | 69 |
| 5 | 3-F | 54 |
| 6 | 4-F | 66 |
| 7 | 3-Cl | 64 |
| 8 | 4-Cl | 66 |
| 9 | 3,5-CF3 | 78 |
| 10 | 3-Me | 51 |
| 11 | 4-Me | 48 |
| 12 | 3-OMe | 61 |
| 13 | 4-OMe | 32 |
| 14 | 2,4,6-Me | 8 |
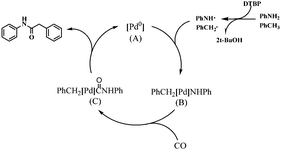
The full mechanistic details of this transformation have not been determined, however, in the presence of the radical scavenger TEMPO the reaction is completely suppressed, indicative of a radical process,28 which is similar to the one proposed by Huang and co-workers for the formation of esters from alkanes and alcohols using a similar catalytic system. A plausible reaction mechanism is shown in Scheme 3. In the presence of a ligand, sequential oxidation of the Pd(0) bis-phosphine catalyst generated in situ with the anilino and benzyl radicals produced in the presence of DTBP leads to the formation of intermediate (B). Subsequent insertion of CO gives intermediate (C) which can undergo reductive elimination to afford the final product. The concentration of aniline strongly influences the yield of the product, i.e. at high concentrations the yield of 1,3-diphenylurea is increased (see ESI‡), presumably because the aniline can more easily react with itself and CO to form 1,3-diphenylurea.
ESI-MS was used to analyze the reaction and a peak that may be tentatively assigned to [(Xantphos)PdCH2Ph]+ (Fig. S1, ESI‡) was observed. This species could be derived from either B or C (see ESI‡ for further details).
In summary, a convenient and efficient method for the synthesis of amides via Pd-catalyzed oxidative carbonylation of C–H bonds with CO has been devised. The method represents a practical and efficient approach for the synthesis of substituted phenyl amides from simple alkanes.
This work was supported by the EPFL and the Swiss National Science Foundation.
Notes and references
- (a) T. Cupido, J. T. Puche, J. Spengler and F. Albericio, Curr. Opin. Drug Discovery Dev., 2007, 10, 768 CAS; (b) J. W. Bode, Curr. Opin. Drug Discovery Dev., 2006, 9, 765 CAS; (c) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243 CrossRef CAS PubMed.
- C. Singh, V. Kumar, U. Sharma, N. Kumar and B. Singh, Curr. Org. Synth., 2013, 10, 241 CrossRef CAS . (The references cited in this publication).
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471 CrossRef CAS PubMed.
- C. L. Allen and J. M. Williams, J. Chem. Soc. Rev., 2011, 40, 3405 RSC.
- C. Gunanathan, Y. B. David and D. Milstein, Science, 2011, 317, 790 CrossRef PubMed.
- (a) A. Brennfhrer, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114 CrossRef PubMed; (b) C. E. Houlden, M. Hutchby, C. D. Bailey, J. G. Ford, N. G. Simon, M. R. Tyler, G. C. Gag, K. I. L. Jones and B. Milburn, Angew. Chem., Int. Ed., 2009, 48, 1830 CrossRef CAS PubMed; (c) R. Giri and J. Q. Yu, J. Am. Chem. Soc., 2008, 130, 14082 CrossRef CAS PubMed; (d) S. Inoue, H. Shiota, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2009, 131, 6898 CrossRef CAS PubMed; (e) R. Lang, L. J. Shi, D. F. Li, C. G. Xia and F. W. Li, Org. Lett., 2012, 14(16), 4130 CrossRef CAS PubMed; (f) A. Takacs, A. Czompa, G. Krajsovszky, P. Matyus and L. Kollar, Tetrahedron, 2012, 68, 7855 CrossRef CAS PubMed.
- B. Gabriele, G. Salerno, L. Veltri and M. Costa, J. Organomet. Chem., 2001, 622, 84 CrossRef CAS.
- Y. Izawa, I. Shimizu and A. Yamamoto, Bull. Chem. Soc. Jpn., 2004, 77, 2033 CrossRef CAS.
- S. I. Lee, S. U. Son and Y. K. Chung, Chem. Commun., 2002, 1310 RSC.
- J. R. Martinelli, D. A. Watson, D. M. M. Freckmann, T. E. Barder and S. L. Buchwald, J. Org. Chem., 2008, 73, 7102 CrossRef CAS PubMed.
- H. Zhang, R. Y. Shi, A. X. Ding, L. J. Lu, B. Chen and A. W. Lei, Angew. Chem., Int. Ed., 2012, 51, 12542 CrossRef CAS PubMed.
- D. D. Liang, Z. W. Hu, J. L. Peng, J. B. Huang and Q. Zhu, Chem. Commun., 2013, 49, 173 RSC.
- (a) K. D. Hesp, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2011, 133, 11430 CrossRef CAS PubMed; (b) Y. Du, T. K. Hyster and T. Rovis, Chem. Commun., 2011, 47, 12074 RSC.
- K. Muralirajan, K. Parthasarathy and C. H. Cheng, Org. Lett., 2012, 14, 4262 CrossRef CAS PubMed.
- J. Tsuji, Palladium Reagents and Catalysts, 2003.
- B. Mandy-Nicole (née Gensow), F. Zoraida and Piet W. N. M. van Leeuwen, Chem. Soc. Rev., 2009, 38, 1099 RSC.
- P. Xie, Y. J. Xie, B. Qian, H. Zhou, C. G. Xia and H. M. Huang, J. Am. Chem. Soc., 2012, 134, 9902 CrossRef CAS PubMed.
- F. M. Miloserdov and V. V. Grushin, Angew. Chem., Int. Ed., 2012, 51, 3668 CrossRef CAS PubMed.
- J. J. Yin and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 6043 CrossRef CAS PubMed.
- H. J. Chen, C. B. Cai, X. H. Liu, X. W. Li and H. F. Jiang, Chem. Commun., 2011, 47, 12224 RSC.
- (a) H. M. Colquhoun, D. J. Thompson and M. V. Twigg, Carbonylation, Direct Synthesis of Carbonyl Compounds, Plenum Press, New York, 1991 Search PubMed; (b) M. Beller, Carbonylation of Benzyl- and Aryl-X Compounds, In Applied Homogeneous Catalysis with Organometallic Compounds, ed. B. Cornils and W. A Herrmann, 2nd edn, Wiley-VCH, Weinheim, 2002 Search PubMed; (c) L. Kollár, Modern carbonylation methods, Wiley-VCH, Verlag GmbH & Co. KgaA, Weinheim, 2008 Search PubMed; (d) C. F. J. Barnard, Organometallics, 2008, 27, 5402 CrossRef CAS; (e) A. Brennfuhrer, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114 CrossRef PubMed; (f) X. F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986 RSC.
- F. Estevan, A. García-Bernabé, P. Lahuerta, M. Sanau, M. A. Ubeda and J. R. Galan-Mascaros, J. Organomet. Chem., 2000, 596, 248 CrossRef CAS.
- C. E. Housecroft, B. A. M. Shaykh, A. L. Rheingold and B. S. Haggerty, Acta Crystallogr., Sect. C, 1990, 46, 1549 CrossRef.
- M. Tromp, J. A. van Bokhoven, G. P. F. van Strijdonck, P. W. N. M. van Leeuwen, D. C. Koningsberger and D. E. Ramaker, J. Am. Chem. Soc., 2004, 127, 777 CrossRef PubMed.
- E. Burello and G. Rothenberg, Adv. Synth. Catal., 2005, 347, 1969 CrossRef CAS.
- T. M. Konrad, J. A. Fuentes, A. M. Z. Slawin and M. L. Clarke, Angew. Chem., Int. Ed., 2010, 49, 9917 CrossRef PubMed.
- Y. R. Luo, Handbook of Bond Dissociation Energies in Organic Compounds, CRC Press, Boca Raton, FL, 2002 Search PubMed.
- L. Boisvert, M. C. Denney, S. K. Hanson and K. I. Goldberg, J. Am. Chem. Soc., 2009, 131, 1580 CrossRef PubMed.
Footnotes |
| † This article is published in celebration of the 50th anniversary of the opening of the Chemistry Department at the University of York. |
| ‡ Electronic supplementary information (ESI) available: Full experimental details including instrumentation, catalytic procedures, mechanistic studies and spectroscopic data. See DOI: 10.1039/c3cc47015f |
| This journal is © The Royal Society of Chemistry 2014 |