Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A tellurium-substituted Lindqvist-type polyoxoniobate showing high H2 evolution catalyzed by tellurium nanowires via photodecomposition†
Jung-Ho
Son
*a,
Jiarui
Wang
a,
Frank E.
Osterloh
a,
Ping
Yu
b and
William H.
Casey
*ac
aDepartment of Chemistry, University of California, Davis, One Shield Ave., Davis, CA 95616, USA. E-mail: junghoson@gmail.com; whcasey@ucdavis.edu; Fax: +1 530 752 8995; Tel: +1 530 752 3211
bKeck NMR Facility, University of California, Davis, One Shields Ave., Davis, CA 95616, USA
cDepartment of Geology, University of California, Davis, One Shield Ave., Davis, CA 95616, USA
First published on 12th November 2013
Abstract
A new tellurium-substituted Lindqvist-type polyoxoniobate [H2TeNb5O19]5− was synthesized as a tetramethylammonium salt. When irradiated with a Xe lamp, a water–methanol solution of this cluster showed exceptionally high H2-evolution activity suggesting cocatalysis by the hexaniobate cluster and metallic tellurium, both of which are formed as photodecomposition products.
The most common aqueous complexes of NbV are the hexaniobate Lindqvist ion (Nb6) and the decaniobate ion and these two clusters dominate in different pH regions.1 Heterometal-substituted Lindqvist-type polyoxoniobate ions are virtually unknown and the single example is the tungsten-rich [HNb2W4O19]3− ion.2 Herein we report a tellurium-monosubstituted Lindqvist-type polyoxoniobate ion as a tetramethylammonium (TMA) salt, TMA5[H2TeNb5O19]·20H2O (1). This molecule is important for two reasons, firstly because 125Te is an NMR-active nucleus with spin 1/2 and a long relaxation time, allowing study of aqueous chemistry in ways that were difficult with the unsubstituted hexaniobate ion, wherein only 17O NMR could be used as an NMR probe to solution chemistry,3 and secondly because the molecule photodecomposes to a catalytic system for H2 production, as shown in this study. Although the niobate Lindqvist ion is well known, its utility in the field of hydrogen energy is not well investigated. Here we demonstrated that Te-substitution of the Lindqvist ion can generate a highly active photocatalytic system in comparison to the pristine Lindqvist hexaniobate ion.
Tellurium substitution has been previously achieved in other polyoxometalate systems including Anderson-type [TeMo6O24]6− clusters, trans-disubstituted Lindqvist-type [Te2Mo4O19]6− clusters and decavanadate-type [HTeV9O28]4− clusters.4,5 A mixed oxide of molybdenum, vanadium, tellurium and niobium has been shown to be a good catalyst for conversion of hydrocarbons; thus the tellurium-substituted niobate ion has potential to act as a catalytically active oxide precursor.4a,5a Solid niobates have also been studied as water-splitting photocatalysts, along with more effective and well-known titanates and tantalates,6 as niobium oxides have a bandgap energy of about 3.4 eV and that compares well to ZnO or TiO2.7 Photocatalytic H2 evolution activities of oligomeric polyoxoniobates have been reported recently.8
The synthesis of 1 was carried out by a hydrothermal reaction of a mixture of Te(OH)6 (telluric acid), hydrous niobium oxide and TMAOH. Electrospray-ionization mass spectrometry (ESI-MS) of the solution after reaction suggested the formation of a new Te-substituted cluster, as proven by the complex fingerprint of each peak due to the naturally occurring Te isotopes and higher m/z values compared to the TMA salt of hexaniobate, TMA5[H3Nb6O19]·20H2O (2) [Fig. S1, S4 and S5, ESI†]. The peak positions match well with calculated peaks of the TMA salt of Te-monosubstituted hexaniobate [Fig. S1, ESI†].
The structure of [H2TeNb5O19]5− (TeNb5) in 1 was determined by X-ray single crystallography [Fig. 1].‡ The tellurium substitution is obvious from the high electron density in one (Te1/Nb1 site) of the three crystallographically independent metal sites. Tellurium is disordered between the two opposite metal sites in the Lindqvist ion due to its centrosymmetry. Refinement with the partial occupancy model at the Te1/Nb1 site showed that the sum of tellurium occupancy in these two sites is 0.95, which is in good agreement with the suggested TeNb5 stoichiometry. Two protons are bound on the two opposite μ2-O, linking Nb2 and Nb3 on the equatorial plane. Five TMA countercations are found in the structure refinement, and the formula is expressed as TMA5[H2TeNb5O19]·20H2O. The cell constants are very similar to those of the previously reported 2,9 which is in agreement with the isostructural and isovalent Lindqvist-type cluster crystallized with the same number of TMA countercations and crystallization water. As a result of the substitution, the terminal Te/Nb–Ot bond (1.8082(12) Å) is longer than the unsubstituted terminal Nb–Ot bond in the periphery (1.7505(11) and 1.7727(11) Å). And the Te/Nb–μ6-O bond (2.28325(15) Å) is shorter than Nb–μ6-O bonds (2.41480(16) and 2.44065(15) Å). Because of the mixed metal occupancy, thermal ellipsoids of Te1/Nb1 and μ6-O are elongated in the axial direction [Fig. 1].
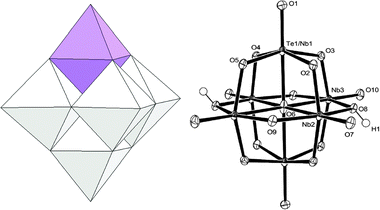 | ||
| Fig. 1 Polyhedral model (left, TeVI: purple, NbV: white) and thermal-ellipsoid model of [H2TeNb5O19]5− (right). Unlabelled atoms are related to the labelled atoms by inversion symmetry. | ||
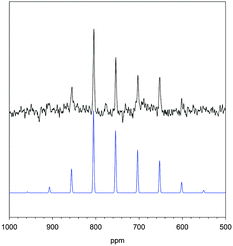
Solid-state 125Te NMR experiment was performed to characterize the TeVI in the cluster. The single substitution in TeNb5 suggests one peak. As predicted, solid-state 125Te NMR spectrum shows one isotropic peak at 754 ppm accompanied with spinning sidebands [Fig. 2]. We note that the spinning sidebands are not symmetric about the central isotropic peak because Te is in an asymmetric structural environment. The Te is located at one metal site of the Lindqvist cluster, which is intrinsically asymmetric, and faces the center of the cluster on one side, while the other side is coordinated with the terminal oxygen atom and is closer to TMA ions and crystallization waters [Fig. 1, Fig. S2, ESI†].
The pH-dependent stabilities of 1 and 2 were examined by performing ESI-MS and UV-Vis titration experiments. ESI-MS spectra of 31 mM solution (pH = 8.1) of each compound were recorded after the addition of acid or base to modify the solution pH. The peaks of 1 only slightly decreased up to pH ∼ 13 when titrated with base, indicating that the TeNb5 cluster is stable under strongly basic conditions [Fig. S4, ESI†]. When titrated with acid, the solution became cloudy, and increasingly so, upon addition of each aliquot below pH 7, which was probably due to formation of hydrous niobium–oxide colloids. However, most of the clusters in solution had converted to colloidal particles at pH ∼ 5 and below. ESI-MS titration of 2 showed similar behaviour [Fig. S5, ESI†]; Nb6 was stable between 7 < pH < 12 but less stable under extreme pH conditions, as suggested by normalized abundance of representative peaks in ESI-MS depending on pH [Fig. S6, ESI†]. In the UV-Vis titration experiment using a more dilute solution (0.03 mM), the electronic spectra of 1 which show LMCT bands at 240 nm and small shoulder at 265 nm did not change significantly up to pH 12.5, which agrees with the stability of TeNb5 under the basic conditions [Fig. S7, ESI†]. During the titration with acid, the overall absorption increased up to pH∼4, which is indicative of decomposition. A solution of 2 showed a similar trend in the UV-Vis titration [Fig. S8, ESI†].
We examined photochemical H2 evolution from each 0.2 g of 1 and 2 in a water–methanol solution (25 mL, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) under UV and visible light from a 300 W Xe lamp. Hydrogen evolution activity of TMA salt of peroxohexaniobate, TMA5[H3Nb6O13(O(I)2)6]·9.5H2O (3),9 was also measured. Methanol was used as a sacrificial electron donor and H2 was monitored by gas chromatography. Visible light (>400 nm) did not produce H2; however, under full Xe spectrum illumination a solution of 1 evolved H2 at a rate of 776 μmol h−1 g−1, which is 70 times higher than that of 2 (11 μmol h−1 g−1) [Fig. 3]. Upon irradiation, the solution of 2 remained colorless while the solution of 1 became gradually darker and about 10 mg of a black precipitate formed after 8 hours, which was identified as metallic tellurium in mostly nanowire morphology mixed with some microcrystals, on the basis of SEM and XRD data (Fig. S9 and S10, ESI†). The formation of metallic tellurium indicates reduction of the TeVI in 1 to Te0 as the cluster decomposed. The apparent chemical conversion of the niobate cluster is also reflected in the non-linear H2 evolution plot, which shows an abrupt increase of activity after 2 h. The main product of the photo-assisted conversion of 1–3 in the solution phase after irradiation was the Nb6 ion, as detected by ESI-MS. The peroxohexaniobate ion 3 showed lowest H2 evolution activity (1 μmol h−1 g−1) when compared to 1 and 2, but evolved small amounts of O2 during irradiation, likely due to the photo-induced removal of the peroxo ligand.
20 v/v) under UV and visible light from a 300 W Xe lamp. Hydrogen evolution activity of TMA salt of peroxohexaniobate, TMA5[H3Nb6O13(O(I)2)6]·9.5H2O (3),9 was also measured. Methanol was used as a sacrificial electron donor and H2 was monitored by gas chromatography. Visible light (>400 nm) did not produce H2; however, under full Xe spectrum illumination a solution of 1 evolved H2 at a rate of 776 μmol h−1 g−1, which is 70 times higher than that of 2 (11 μmol h−1 g−1) [Fig. 3]. Upon irradiation, the solution of 2 remained colorless while the solution of 1 became gradually darker and about 10 mg of a black precipitate formed after 8 hours, which was identified as metallic tellurium in mostly nanowire morphology mixed with some microcrystals, on the basis of SEM and XRD data (Fig. S9 and S10, ESI†). The formation of metallic tellurium indicates reduction of the TeVI in 1 to Te0 as the cluster decomposed. The apparent chemical conversion of the niobate cluster is also reflected in the non-linear H2 evolution plot, which shows an abrupt increase of activity after 2 h. The main product of the photo-assisted conversion of 1–3 in the solution phase after irradiation was the Nb6 ion, as detected by ESI-MS. The peroxohexaniobate ion 3 showed lowest H2 evolution activity (1 μmol h−1 g−1) when compared to 1 and 2, but evolved small amounts of O2 during irradiation, likely due to the photo-induced removal of the peroxo ligand.
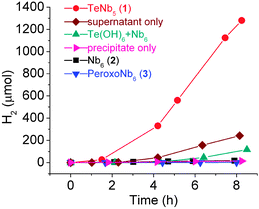 | ||
| Fig. 3 Hydrogen evolution data in 20% (v/v) methanol–water solution (25 mL) during the irradiation from full spectrum of Xe lamp. | ||
The supernatant and precipitate were separated after irradiation of solution of 1 for 8 h to evaluate the H2-evolution activity of each photo-decomposition product separately. Te0 wires alone showed very low H2 evolution activity when mixed with water–methanol [Fig. 3]. The separated supernatant continued to form more Te0 upon irradiation due to the small amount of tellurate present in the supernatant. This supernatant showed a similar H2 evolution curve but lower H2 evolution activity than the original solution [Fig. 3] probably due to the smaller amount of precipitate, suggesting that coexistence of the niobate cluster and metallic Te0 is necessary to maintain the high H2 evolution activity.
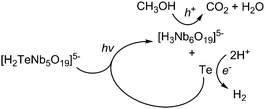
These results suggest a mechanism for photocatalytic H2 evolution shown in Scheme 1. Photoexcitation of TeNb5 forms an electron–hole pair and the electron reduces TeVI in the cluster. As a result, TeNb5 is decomposed to Te0 and Nb6 with the hole oxidizing methanol as a sacrificial electron donor, ultimately forming CO2 and H2O. Once TeVI is fully reduced to Te0, it may further accept electrons to become telluride (Te2−). We speculate that this aqueous telluride will reduce protons to evolve H2, as H2Te has been shown to decompose to metallic tellurium in aqueous solution.10 Thus we believe that the high H2 evolution activity in this system is due to coexistence of Nb6 and Te0 nanowires or colloids and that the native tellurium metal acts as a H2-evolution cocatalyst. Cobaloxime or H2PtCl6 has been commonly used as a cocatalyst in the study of H2 evolution from polyoxoniobate and many other systems.6c,8 To our knowledge, this is the first demonstration of Te0 as a H2 evolution cocatalyst.
For control experiments, stoichiometric mixtures of telluric acid and 2 (Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb = 1
Nb = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were tested for H2 evolution. Nearly the same amount (∼10 mg) of Te0 formed in this solution after irradiation, but the amount of H2 evolved (71 μmol h−1 g−1) was about an order of magnitude smaller than that produced from the solution of 1, but higher than that produced from the solution of 2 alone [Fig. 3]. This result implies that the reduction of TeVI in TeNb5 can occur directly within the cluster from the photogenerated electron–hole pairs, in such a way that formation of metallic tellurium in the solution of 1 is faster than in the simple solution mixture of telluric acid and 2. This explains the high H2 evolution rate in the early stage of irradiation of 1 compared to the control experiment. The total surface area of the particles in the solution of 1 might also be higher than that in the mixture of telluric acid and 2 because of the faster formation of particles, which might have resulted in the high H2-evolution activity.
5) were tested for H2 evolution. Nearly the same amount (∼10 mg) of Te0 formed in this solution after irradiation, but the amount of H2 evolved (71 μmol h−1 g−1) was about an order of magnitude smaller than that produced from the solution of 1, but higher than that produced from the solution of 2 alone [Fig. 3]. This result implies that the reduction of TeVI in TeNb5 can occur directly within the cluster from the photogenerated electron–hole pairs, in such a way that formation of metallic tellurium in the solution of 1 is faster than in the simple solution mixture of telluric acid and 2. This explains the high H2 evolution rate in the early stage of irradiation of 1 compared to the control experiment. The total surface area of the particles in the solution of 1 might also be higher than that in the mixture of telluric acid and 2 because of the faster formation of particles, which might have resulted in the high H2-evolution activity.
In conclusion, the TMA salt of the TeNb5 molecule reported herein exhibits a similar pH stability window and solid-state structure compared to Nb6, but demonstrates much higher H2-evolution activity via a photo-decomposition route to Nb6 and Te0 nanowires. Future studies shall focus on the hydrolysis and isotope-exchange kinetics of the TeNb5 molecule, by using 17O and 125Te NMR together, and compare hydrolysis chemistry with that of the unsubstituted Nb6.
This work was supported by an NSF CCI grant through the Center for Sustainable Materials Chemistry, number CHE-1102637.
Notes and references
- (a) I. Lindqvist, Ark. Kemi, 1953, 5, 247–250 CAS; (b) E. J. Graeber and B. Morosin, Acta Crystallogr., Sect. B, 1977, 33, 2137–2143 CrossRef; (c) S. Si Larbi, D. Bodiot and B. Spinner, Rev. Chim. Miner., 1976, 13, 497–507 CAS; (d) A. Goiffon and B. Spinner, Tantala, 1977, 24, 130–132 CrossRef CAS; (e) M. Nyman, Dalton Trans., 2011, 40, 8049–8058 RSC.
- V. W. Day, W. C. Klemperer and C. Schwartz, J. Am. Chem. Soc., 1987, 109, 6030–6044 CrossRef CAS.
- (a) T. M. Alam, M. Nyman, B. R. Cherry, J. M. Segall and L. E. Lybarger, J. Am. Chem. Soc., 2004, 126, 5610–5620 CrossRef CAS PubMed; (b) J. R. Black, M. Nyman and W. H. Casey, J. Am. Chem. Soc., 2006, 128, 14712–14720 CrossRef CAS PubMed.
- (a) I. L. Botto, C. I. Cabello and H. J. Thomas, Mater. Chem. Phys., 1997, 47, 37–45 CrossRef CAS; (b) P. A. Lorenzo Luis, P. Martín-Zarza, A. Sanchez, C. Ruiz-Pérez, M. Hernández-Molina, X. Solans and P. Gili, Inorg. Chim. Acta, 1998, 277, 139–150 CrossRef CAS; (c) D. Drewes, E. M. Limanski and B. Krebs, Eur. J. Inorg. Chem., 2004, 4849–4853 CrossRef CAS; (d) D. Drewes and B. Krebs, Z. Anorg. Allg. Chem., 2005, 631, 2591–2594 CrossRef CAS; (e) J. Wang, G. Zhang, P. Ma and J. Niu, Inorg. Chem. Commun., 2008, 11, 825–828 CrossRef CAS PubMed.
- (a) R. I. Maksimovskaya, V. M. Bondareva and G. I. Aleshina, Eur. J. Inorg. Chem., 2008, 4906–4914 CrossRef CAS; (b) S. Konaka, Y. Ozawa and A. Yagasaki, Inorg. Chem. Commun., 2008, 11, 1267–1269 CrossRef CAS PubMed; (c) S. Konaka, Y. Ozawa, T. Shonaka, S. Watanabe and A. Yagasaki, Inorg. Chem., 2011, 50, 6183–6188 CrossRef CAS PubMed.
- (a) Y. Ebina, A. Tanaka, J. N. Kondo and K. Domen, Chem. Mater., 1996, 8, 2534–2538 CrossRef CAS; (b) A. Kudo, H. Kato and S. Nakagawa, J. Phys. Chem. B, 2000, 104, 571–575 CrossRef CAS; (c) F. Osterloh, Chem. Mater., 2008, 20, 35–54 CrossRef CAS; K. Akatsuka, G. Takanashi, Y. Ebina, M. Haga and T. Sasaki, J. Phys. Chem. C, 2012, 116, 12426–12433 Search PubMed.
- A. G. S. Prado, L. B. Bolzon, C. P. Pedroso, A. O. Moura and L. L. Costa, Appl. Catal., B, 2008, 82, 219–224 CrossRef CAS PubMed.
- (a) Z. Zhang, Q. Lin, D. Kurunthu, T. Wu, F. Zuo, S.-T. Zheng, C. J. Bardeen, X. Bu and P. Feng, J. Am. Chem. Soc., 2011, 133, 6934–6937 CrossRef CAS PubMed; (b) P. Huang, C. Qin, Z.-M. Su, Y. Xing, X.-L. Wang, K.-Z. Shao, Y.-Q. Lan and E.-B. Wang, J. Am. Chem. Soc., 2012, 134, 14004–14010 CrossRef CAS PubMed; (c) Z.-L. Wang, H.-Q. Tan, W.-L. Chen, Y.-G. Li and E.-B. Wang, Dalton Trans., 2012, 41, 9882–9884 RSC.
- C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 8251–8254 CrossRef CAS PubMed.
- L. M. Dennis and R. P. Anderson, J. Am. Chem. Soc., 1914, 36, 882–909 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis details, instrumental analysis conditions, ESI-MS spectrum of 1, crystal packing diagram, FT-IR spectra of 1, 2 and 3, pH dependent ESI-MS and UV-Vis spectra of 1 and 2, SEM and XRD of tellurium precipitate. CCDC 959006. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc47001f |
‡ Crystal data. (1) CCDC 959006. C20H94N5O39.25TeNb5, M = 1625.15, monoclinic, a = 33.8700(15) Å, b = 8.4954(4) Å, c = 21.9238(10) Å, β = 110.475(1)°, U = 5909.8(5) Å3, T = 93 K, space group C2/c (no. 15), Z = 4, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 567 reflections measured, 6782 unique (Rint = 0.0218) which were used in all calculations. The final wR(F2) was 0.0407 (all data). 567 reflections measured, 6782 unique (Rint = 0.0218) which were used in all calculations. The final wR(F2) was 0.0407 (all data). |
| This journal is © The Royal Society of Chemistry 2014 |