Water swelling, brine soluble imidazole based zwitterionic polymers – synthesis and study of reversible UCST behaviour and gel–sol transitions†
Vivek Arjunan Vasantha*a,
Satyasankar Janaa,
Anbanandam Parthiban*a and
Julius G. Vancsoab
aInstitute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 1 Pesek Road, Jurong Island, Singapore 627833. E-mail: vivek_vasantha@ices.a-star.edu.sg; aparthiban@ices.a-star.edu.sg
bMESA+ Research Institute for Nanotechnology, Faculty of Science and Technology, University of Twente, P. O. Box 217, 7500 AE Enschede, The Netherlands
First published on 18th July 2013
Abstract
New vinylbenzene substituted imidazole based zwitterionic polymers with unique characteristics like swelling in water and solubility in concentrated brine solution in which they exhibited a reversible upper critical solution temperature (UCST) and gel–sol transitions are reported herein.
Zwitterionic polymers,1–3 such as polybetaines, have gained much importance of late due to their wide applications in antifouling,4–6 membranes,7 biotechnology,8–15 enhanced oil recovery (EOR),16 as coatings,4,17 etc. The three main classes of zwitterionic polymers developed so far include carboxy betaines, phosphobetaines and sulfobetaines. Among these three classes, sulfobetaines are the most convenient to synthesize3,18 and thus employed widely.19 Sulfobetaines derived from acrylamide and methacrylates (Scheme 1) are two of the prominently used monomers for making zwitterionic polymers which are also commercially available.20–22 However, these monomers are hydrolytically unstable. These monomers undergo hydrolysis to varying degrees even during polymerization of the zwitterionic monomers resulting in the formation of poly(acrylic acid) along with the zwitterionic polymer. For example, the ester based zwitterionic monomer (Scheme 1a) undergoes hydrolysis to the extent of 12–15%, and 8% of the amide derived zwitterionic monomer (Scheme 1b) is hydrolyzed during polymerization.23 One of the important applications of these zwitterionic monomers is to prepare polymer brushes, which are potentially useful for making antifouling biomedical devices.24,25 Thus such polymer brushes are not only contaminated but are also vulnerable to lose the brush nature sooner due to hydrolysis. The unique hydration characteristics which are the source of special properties such as antifouling will also disappear with the loss of the brush nature as a result of hydrolysis.
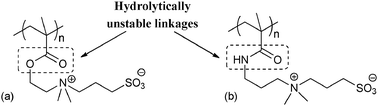 | ||
| Scheme 1 Chemical structure of zwitterionic (a) poly(methyl methacrylate) and (b) poly(methyl methacrylamide). | ||
With the aim of synthesizing polysulfobetaines free from hydrolyzable linkages we recently designed and synthesized imidazole based sulfobetaine monomers (Scheme 2 and Scheme S2, ESI†).
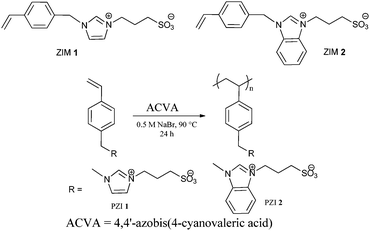 | ||
| Scheme 2 Chemical structures and polymerization of newly synthesized zwitterionic monomers (ZIMs). | ||
As reported herein, these imidazole based polysulfobetaines exhibited many unusual solubility characteristics. Unlike the acrylate and acrylamide derived polysulfobetaines which are soluble in water, the benzimidazole based polysulfobetaines are only soluble in concentrated brine solution (22.6 wt% NaCl) and also swell in deionized water. We believe such solubility characteristics are potentially useful for applications like EOR which is gaining significance due to depleting petroleum reserves. These imidazole derived polymeric zwitterions are unique in that quite apart from the ionic nature they also exhibit other interactions like donor–acceptor as well as comparatively more hydrophobicity than the conventional sulfobetaines. This characteristic is the source of the difference in solubility as described below.
The precursors ZIM 1 and 2 were synthesized by reacting vinylbenzylchloride with imidazole and benzimidazole, respectively, followed by a nucleophilic addition reaction with 1,3 propane sultone in good yield (65–74%). To the best of our knowledge, ZIM 1 and 2 have not been reported before. The monomers ZIM 1 and 2 were obtained as hygroscopic powders, which were characterized by NMR, FTIR, ESI-MS, and elemental analysis (Fig. S1, ESI†). The characterization results are summarized in Table S1 (ESI†).
ZIMs were polymerized at 90 °C using 4,4′-azobis(4-cyanovaleric acid) (ACVA) as the initiator in aqueous sodium bromide solution (25 wt%) as shown in Scheme 2. The polymerization results are summarized in Table 1. The polymeric zwitterions, PZIs, were characterized using 1H NMR, FTIR, GPC, and UV-Vis spectroscopy (Fig. S2–S5, ESI†). These studies have confirmed that the zwitterionic nature is unaffected during polymerization. The solubility of PZIs was tested in various solvents as detailed in Table S2 (ESI†).
| Monomer/initiator (mmol) | Time | Yielda | Mn,GPC (g mol−1) | PDI | Elemental analysis |
|---|---|---|---|---|---|
| a Determined by gravimetry after drying the polymer at 50 °C in vacuo. | |||||
| ZIM 1 (3.29)/ACVA (0.0165) | 1.5 h | 98% | 6500 | 2.02 | Calc. N, 8.13; S, 9.31; Exp. N, 7.78; S, 9.00 |
| ZIM 2 (2.81)/ACVA (0.0189) | 24 h | >98% | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.40 | Calc. N, 5.29; S, 6.05; Exp. N, 5.14; S, 6.04 |
PZI 1 and 2 behaved like hydrogels and the equilibrium swelling ratio measured in deionized water is shown in Fig. S6 (ESI†). The swelling tendency and the lack of solubility in deionized water in spite of their zwitterionic character are due to the comparatively higher hydrophobic nature as well as due to strong donor–acceptor type interactions. The hydration studies of PZIs are summarized in Table 2.
| Sample | Swelling ratio (SR, %) | Critical salt concentration (CSC, wt%) | ||
|---|---|---|---|---|
| Titration | Transmittance | DLS | ||
| a SR = [(weight of swollen polymer − weight of dry polymer)/weight of dry polymer] × 100. For CSC, 0.9 wt% of polymers were dissolved in 22.62 wt% of NaCl solution and titrated with deionized water at 25 °C (Fig. S6, ESI). | ||||
| PZI 1 | 85.23 ± 4.26 | 1.71 ± 0.2 (0.17 M) | 1.52 | 1.74 |
| PZI 2 | 134.76 ± 6.74 | 7.67 ± 0.2 (1.31 M) | 7.82 | 8.20 |
The addition of salt readily enhances the solubility of PZIs in water, mainly due to the “anti polyelectrolyte” effect.2 The effect of the amount of salt on the solubility of these polymers, i.e. the minimum (critical) salt concentration (CSC) necessary to dissolve the polymers, was determined by volumetric titration, transmittance and dynamic light scattering (DLS) studies as shown in Fig. S7 (ESI†). The presence of an additional benzene ring in PZI 2 induces a significant solubility difference as shown in Table 2. The CSC for PZI 2 is higher than that for the other sulfobetaines reported in the literature.26–28 In comparison, the acrylamide based sulfobetaine polymer formed a clear solution at 0.25 wt% of NaCl solution and formed an opaque gel in NaCl solution at a concentration of 27.8 wt% at 20 °C.29 A combination of factors such as rigid structures, which could shorten the Debye length, additional donor–acceptor interactions, increased hydrophobicity, etc. are responsible for this change. The unusual solubility behaviour at high concentrations of salt solution and a lower pKa (Table S3, ESI†) of PZIs could be indicative of a more stable sulfonate anion.30 The stability towards pH 2 to pH 13 makes PZIs potentially applicable for EOR16 and antifouling.31
Aqueous salt solution also promoted the temperature-dependent phase separation of PZIs. Both PZIs exhibited reversible UCST phase transition32,33 behaviour between 5 °C and 95 °C in NaCl solution (12.13–5.1 wt% NaCl solution for PZI 2). As illustrated in Fig. 1, the polyzwitterions reached a maximum UCST > 95 °C at low salt concentrations (5.1 wt% NaCl). Fig. S7 (ESI†) shows the phase transition (UCST) of PZI 1 and 2 as a function of NaCl concentration with respect to transmittance observed visually by the cloudy appearance of the solution. The UCST of PZIs was tuneable by changing the salt and polymer concentration. The reversible thermal responsive behaviour of zwitterionic polymers during heating and cooling was also evidenced by DLS (Fig. 1b) and viscosity studies.
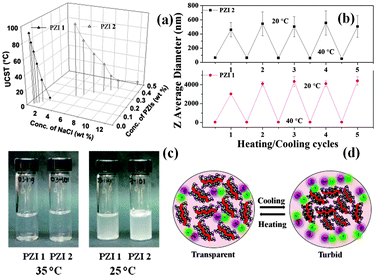 | ||
Fig. 1 UCST of PZIs (a) PZI 1 and PZI 2 in NaCl solution during heating; (b) temperature dependence of the Z average diameter of PZI 1 and 2 in NaCl solution during heating and cooling cycles.  : PZI 1 (0.07 wt% of PZI 1 in 1.62 wt% NaCl) is 39.14 ± 0.097 nm at 40 °C and 3986 ± 559 nm at 20 °C; ■: PZI 2 (0.31 wt% of PZI 2 in 7.89 wt% NaCl) is 63.39 ± 2.44 nm at 40 °C and 514 ± 38.2 nm at 20 °C. Each data point was obtained after equilibrating for 10 min (Fig. S8, ESI†); (c) photographs of PZIs 1 and 2 at variable temperatures and (d) schematic representation of reversible UCST thermal phase transitions. : PZI 1 (0.07 wt% of PZI 1 in 1.62 wt% NaCl) is 39.14 ± 0.097 nm at 40 °C and 3986 ± 559 nm at 20 °C; ■: PZI 2 (0.31 wt% of PZI 2 in 7.89 wt% NaCl) is 63.39 ± 2.44 nm at 40 °C and 514 ± 38.2 nm at 20 °C. Each data point was obtained after equilibrating for 10 min (Fig. S8, ESI†); (c) photographs of PZIs 1 and 2 at variable temperatures and (d) schematic representation of reversible UCST thermal phase transitions. | ||
Above the UCST, a transparent solution was formed, and no aggregate was detected by DLS (Fig. S8 and S9, ESI†). The mono modal peak observed at high temperatures indicates that the polymer may exist as a coil of globular conformation1,33 or may exhibit micelle behaviour.34 In the micelles, the backbone of PZIs made up of hydrophobic polystyrene may act as a core and the hydrophilic zwitterionic moiety could function as a shell. The interaction between the zwitterionic polymer and the salt at UCST was reversible during heating and cooling cycles. Upon heating the solution to 40 °C the turbidity disappeared completely, which reappeared upon cooling to 20 °C due to the formation of aggregates as illustrated in Fig. 1c. Furthermore, the experiment confirmed the reversible thermo responsive behaviour of PZIs.
The viscosity was studied at UCST of PZI 1 and 2 as shown in Fig. S10 and S11 (ESI†). Both PZI 1 and 2 exhibited non-Newtonian behaviour. The intermolecular association between charged species of the zwitterionic polymer is assumed to be responsible for the shear thickening behaviour (dilatancy). With increasing temperature, the viscosity of the solution dropped rapidly, though the rate of change was different with a larger change observed for PZI 1 which may be because of less interaction between polymer chains as well as lower rigidity of the polymer backbone combined with its low molecular weight.
Zwitterionic polymers (PZI 1 and 2) acted as reversible, temperature-sensitive gels in brine solution. Similar to agarose and gelatin, the polymer gels flow upon heating above the critical temperature (Tc,gel). This has been attributed to the disruption of the equilibrium existing among salt, water and zwitterions. Upon cooling, the equilibrium reverted back to the more stable configuration. This gel–sol and sol–gel cycle was repeated several times without any hysteresis. PZI 2 displayed superior gelation properties in that a transparent gel formed at a low concentration of the polymer (3 wt% vs. 42.34 wt% for PZI 1). Fig. 2 shows the thermoreversiblity (gel–sol) of PZI 2 gel. The gel window became wider and shifted to a higher temperature when the polymer concentration was increased from 9 to 16 wt%. The PZI 1 also showed similar gel–sol phase transition behaviour, and a detailed study is in progress.
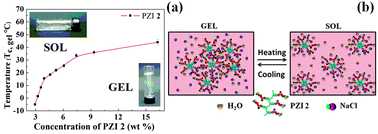 | ||
| Fig. 2 Gel–sol transition behaviour of PZI 2 in 22.6 wt% NaCl (a) and schematic representation of sol–gel behaviour (b). | ||
In summary, new hydrolytically stable imidazole based sulfobetaine zwitterionic polymers were synthesized. The PZIs exhibited reversible phase transitions due to the formation of expanded chains at elevated temperature and aggregates below the UCST as studied by DLS, transmittance and viscosity measurements. The unique thermoreversible gelling properties as well as unusual solubility characteristics make them interesting materials for a wide range of applications. Work is in progress to make polymer brushes from these novel vinylbenzene substituted imidazole based sulfobetaines.
This work was funded by Agency for Science, Technology and Research (A*STAR), Singapore under Innovative Marine Antifouling Solutions (IMAS) for high value applications programme. The authors thank Dr R. Krishnan, Ms Chen Junhui and Ms Foo Ming Choo for assisting in GPC and DLS studies.
Notes and references
- S. E. Kudaibergenov, Polymer Latexes – Epoxide Resins – Polyampholytes, 1999, vol. 144, pp. 115–197 Search PubMed.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177–4189 CrossRef CAS PubMed.
- M. Mertoglu, S. Garnier, A. Laschewsky, K. Skrabania and J. Storsberg, Polymer, 2005, 46, 7726–7740 CrossRef CAS PubMed.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347–4390 CrossRef CAS PubMed.
- A. B. Lowe, M. Vamvakaki, M. A. Wassall, L. Wong, N. C. Billingham, S. P. Armes and A. W. Lloyd, J. Biomed. Mater. Res., 2000, 52, 88–94 CrossRef CAS.
- Z. Zhang, J. A. Finlay, L. Wang, Y. Gao, J. A. Callow, M. E. Callow and S. Jiang, Langmuir, 2009, 25, 13516–13521 CrossRef CAS PubMed.
- F. Q. Xuan and J. S. Liu, Polym. Int., 2009, 58, 1350–1361 CrossRef CAS.
- H. Jiang, X. B. Wang, C. Y. Li, J. S. Li, F. J. Xu, C. Mao, W. T. Yang and J. Shen, Langmuir, 2011, 27, 11575–11581 CrossRef CAS PubMed.
- Y. J. Shih, Y. Chang, A. Deratani and D. Quemener, Biomacromolecules, 2012, 13, 2849–2858 CrossRef CAS PubMed.
- S. H. Ye, C. A. Johnson Jr., J. R. Woolley, H. Murata, L. J. Gamble, K. Ishihara and W. R. Wagner, Colloids Surf., B, 2010, 79, 357–364 CrossRef CAS PubMed.
- J. Zhang, J. Yuan, Y. L. Yuan, J. Shen and S. C. Lin, Colloids Surf., B, 2003, 30, 249–257 CrossRef CAS.
- S. L. West, J. P. Salvage, E. J. Lobb, S. P. Armes, N. C. Billingham, A. L. Lewis, G. W. Hanlon and A. W. Lloyd, Biomaterials, 2004, 25, 1195–1204 CrossRef CAS PubMed.
- P. S. Liu, Q. Chen, X. Liu, B. Yuan, S. S. Wu, J. Shen and S. C. Lin, Biomacromolecules, 2009, 10, 2809–2816 CrossRef CAS PubMed.
- W. H. Kuo, M. J. Wang, H. W. Chien, T. C. Wei, C. Lee and W. B. Tsai, Biomacromolecules, 2011, 12, 4348–4356 CrossRef CAS PubMed.
- Z. Cao, L. Zhang and S. Jiang, Langmuir, 2012, 28, 11625–11632 CrossRef CAS PubMed.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Prog. Polym. Sci., 2011, 36, 1558–1628 CrossRef CAS PubMed.
- T. Mérian and J. M. Goddard, J. Agric. Food Chem., 2012, 60, 2943–2957 CrossRef PubMed.
- L. Sonnenschein and A. Seubert, Tetrahedron Lett., 2011, 52, 1101–1104 CrossRef CAS PubMed.
- S. Y. Jiang and Z. Q. Cao, Adv. Mater., 2010, 22, 920–932 CrossRef CAS PubMed.
- C. L. McCormick and L. C. Salazar, Polymer, 1992, 33, 4617–4624 CrossRef CAS.
- A. T. Nguyen, J. Baggerman, J. M. J. Paulusse, C. J. M. van Rijn and H. Zuilhof, Langmuir, 2011, 27, 2587–2594 CrossRef CAS PubMed.
- H. Kitano, M. Imai, K. Sudo and M. Ide, J. Phys. Chem. B, 2002, 106, 11391–11396 CrossRef CAS.
- P. Mary, D. D. Bendejacq, M.-P. Labeau and P. Dupuis, J. Phys. Chem. B, 2007, 111, 7767–7777 CrossRef CAS PubMed.
- C. J. Huang, Y. T. Li, J. B. Krause, N. D. Brault and S. Y. Jiang, Macromol. Rapid Commun., 2012, 33, 1003–1007 CrossRef CAS PubMed.
- Y. K. Jhon, S. Arifuzzaman, A. E. Ozcam, D. J. Kiserow and J. Genzer, Langmuir, 2012, 28, 872–882 CrossRef CAS PubMed.
- J. C. Salamone, W. Volksen, A. P. Olson and S. C. Israel, Polymer, 1978, 19, 1157–1162 CrossRef CAS.
- T. A. Wielema and J. B. F. N. Engberts, Eur. Polym. J., 1990, 26, 639–642 CrossRef CAS.
- V. M. Monroy Soto and J. C. Galin, Polymer, 1984, 25, 254–262 CrossRef.
- J. Ning, K. Kubota, G. Li and K. Haraguchi, React. Funct. Polym., 2013, 73, 969–978 CrossRef CAS PubMed.
- L. Wu, J. Jasinski and S. Krishnan, J. Appl. Polym. Sci., 2012, 124, 2154–2170 CrossRef CAS.
- D. M. Yebra, S. Kiil and K. Dam-Johansen, Prog. Org. Coat., 2004, 50, 75–104 CrossRef CAS PubMed.
- D. N. Schulz, D. G. Peiffer, P. K. Agarwal, J. Larabee, J. J. Kaladas, L. Soni, B. Handwerker and R. T. Garner, Polymer, 1986, 27, 1734–1742 CrossRef CAS.
- J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898–1920 CrossRef CAS PubMed.
- A. B. Lowe, N. C. Billingham and S. P. Armes, Macromolecules, 1999, 32, 2141–2148 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental description and results including 1H NMR, GPC, DLS, detailed phase studies etc. See DOI: 10.1039/c3cc44407d |
| This journal is © The Royal Society of Chemistry 2014 |
