Photo-regulation of constitutive gene expression in living cells by using ultrafast photo-cross-linking oligonucleotides†
Takashi
Sakamoto
a,
Atsuo
Shigeno
a,
Yuichi
Ohtaki
a and
Kenzo
Fujimoto
*ab
aSchool of Materials Science, Japan Advanced Institute of Science and Technology, 1-1 Asahi-dai, Nomi, Ishikawa 923-1292, Japan. E-mail: kenzo@jaist.ac.jp
bResearch Center for Bio-Architecture, Japan Advanced Institute of Science and Technology, 1-1 Asahi-dai, Nomi, Ishikawa 923-1292, Japan
First published on 26th June 2014
Abstract
3-Cyanovinylcarbazole nucleoside (CNVK), which has an ultrafast photo-cross-linking ability to complementary RNA, was incorporated into the antisense oligonucleotide targeting green fluorescent protein (GFP) mRNA. We successfully demonstrated that the photoreactive antisense oligonucleotides effectively regulate GFP expression in a stable cell line expressing the GFP gene in a photo-responsive manner.
The development of methods for regulating gene expression is one of the most important issues in the field of drug discovery, biology and chemical biology. An antisense strategy1 that can regulate specifically gene expression with the specific binding property of a short oligonucleotide, i.e. an antisense oligonucleotide (AS-ODN), and its complementary target mRNA is a promising technology in this field. In this strategy, chemically modified AS-ODNs, such as phosphorothioates,2 peptide nucleic acids3 and locked (bridged) nucleic acids,4 have been used to avoid the degradation by nucleases in blood or cells or to stabilize the hetero-duplex of the AS-ODN and its target mRNA, and these AS-ODNs successfully regulate the gene expression in vivo.5
As yet another class of chemically modified AS-ODNs, photoresponsive AS-ODNs having a photo-cleavable linker,6 an azobenzene7 or a psolaren moiety8 that can control the stability of the AS-ODN/mRNA hetero-duplex by photo-irradiation have been developed. Based on the photoresponsive manner of these AS-ODNs, regulation of gene expression can be induced with the desired timing and position. Although these AS-ODNs are a promising tool for biochemistry and chemical biology, there are few reports on the photo-regulation of constitutive gene expression. In many cases, photo-regulation of gene expression has been demonstrated using a transient gene expression system with co-transfection of a reporter gene and AS-ODNs.
In this study, we adopted the phosphorothioate AS-ODN having 3-cyanovinylcarbazole nucleoside (CNVK),9 which has an ultrafast photo-cross-linking ability to the pyrimidine base in the target DNA or RNA strand (Fig. 1), as a photo-cross-linker toward green fluorescent protein (GFP) mRNA. The photoresponsive gene silencing activity of these AS-ODNs for the regulation of constitutive GFP expression in a GFP stable cell line (GFP–HeLa) was evaluated (Fig. 2).
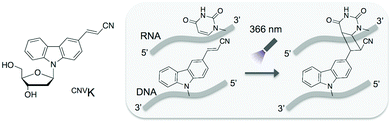 | ||
| Fig. 1 Structure of CNVK and the photo-cross-linking reaction of CNVK with U in DNA/RNA heteroduplex. | ||
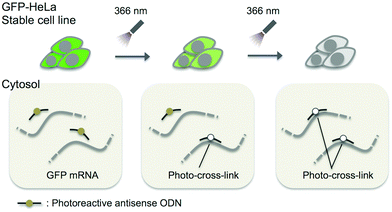 | ||
| Fig. 2 Schematic drawing of the photo-regulation of GFP gene expression in a stable cell line with K-AS. | ||
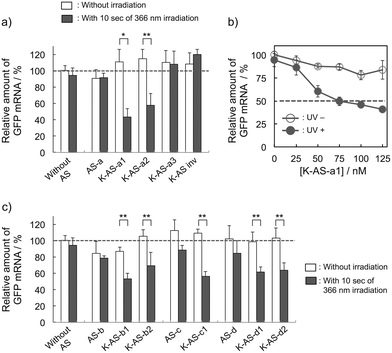
First, the photoreactivities of AS-ODNs (K-AS-a1–3; the sequences of all AS-ODNs are listed in Table 1) toward complementary oligoRNA were estimated by denaturing PAGE analysis (Fig. S1†). In the case of K-AS-a1 and a2, a band identical to the photo-cross-linked dimer appeared by 366 nm photoirradiation, whereas the band did not appear in the case of K-AS inv having an inverted sequence of K-AS-a1, suggesting that the photo-cross-linking reaction occurred in a sequence selective manner. The photo-cross-linked dimer did not appear also in the case of K-AS-a3 having a sequence designed to possess the guanine base at the photo-cross-linking site in the target RNA strand, suggesting that the pyrimidine selectivity of the photo-cross-linking reaction, previously reported,9a was not affected by the phosphorothioate internucleoside modification.
| Name | Target region | Sequencea |
|---|---|---|
| a “X” indicates CNVK. | ||
| AS-a | 14–38 | ACCACCCCGGTGAACAGCTCCTCGC |
| K-AS-a1 | 14–38 | AXCACCCCGGTGAACAGCTCCTCGC |
| K-AS-a2 | 14–38 | ACCACCCCGGTGAXCAGCTCCTCGC |
| K-AS-a3 | 14–38 | ACCACXCCGGTGAACAGCTCCTCGC |
| K-AS inv | 14–38 | CGCTCCTCGACAAGTGGCCCCACXA |
| AS-b | 471–495 | AGTTCACCTTGATGCCGTTCTTCTG |
| K-AS-b1 | 471–495 | AXTTCACCTTGATGCCGTTCTTCTG |
| K-AS-b2 | 471–495 | AGTTCACCTTGAXGCCGTTCTTCTG |
| AS-c | 542–566 | ATGGGGGTGTTCTGCTGGTAGTGGT |
| K-AS-c1 | 542–566 | AXGGGGGTGTTCTGCTGGTAGTGGT |
| AS-d | 592–616 | ACTGGGTGCTCAGGTAGTGGTTGTC |
| K-AS-d1 | 592–616 | AXTGGGTGCTCAGGTAGTGGTTGTC |
| K-AS-d2 | 592–616 | ACTGGGTGCTCAXGTAGTGGTTGTC |
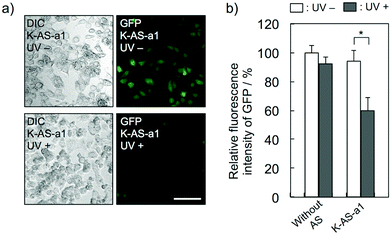
To evaluate the gene silencing activity of photoreactive AS-ODNs, AS-a, K-AS-a1–a3 and K-AS inv (100 nM) were transfected to GFP–HeLa cells, which express continuously the GFP gene. After 10 s of photoirradiation followed by 2 h of incubation, total RNA was extracted and then the expression level of GFP mRNA was quantified by quantitative RT-PCR. As shown in Fig. 3a, only in the case of K-AS-a1 and 2, significant down-regulation of GFP mRNA was induced by 366 nm photoirradiation, although the change in expression level was not observed in the case of AS-a, K-AS-a3 and K-AS inv. These results were consistent with the photo-cross-linking ability of each K-AS ODN discussed above, suggesting that the photo-cross-linking reaction is the key reaction of this antisense-based photoregulation of constitutive GFP expression. As shown in Fig. 3b, the photo-regulation efficiency varies in a dose dependent manner, and the 50% inhibition concentration (IC50) was 75 nM. The IC50 was 2- to 3-fold smaller than that of standard phosphorothioate antisense ODNs for GFP,10 suggesting that the irreversible covalent bond formation between K-AS ODNs and target mRNA enhances the antisense effect. The other K-ASs targeting other regions of GFP mRNA (K-AS-b, c, d) show the same levels of gene silencing effect as K-AS-a (Fig. 3c), although the secondary structure of these target regions would be different from each other (Fig. S2†), suggesting the possibility that the K-AS invade the intramolecular duplex of the target region. The same strand invasion effect was observed in the case of a photoreactive molecular beacon having CNVK.9i As shown in Fig. 4a, b, the fluorescence intensity of GFP in cells was clearly decreased by 40% by the photoirradiation with K-AS-a1 treatment, suggesting that K-AS-a1 can down-regulate the cellular synthesis of GFP by 10 s of photoirradiation. Since the expression levels of β-actin, which is one of the representative endogenous housekeeping genes, and SIN3B, whose mRNA has highest homology (83%) with AS-a among the human genome, were not affected by K-AS-a1 treatment and UV irradiation (Fig. S3†), the K-AS can photo-regulate specifically target gene expression. The 10 s of photoirradiation scarcely affected the viability of GFP–HeLa cells (Fig. S4a†) and the amount of pyrimidine (6–4) pyrimidone photoproducts (Fig. S4b†), which is one of the representative pyrimidine photodimers caused by UV irradiation, suggesting that photodamage caused by the irradiation was insignificant.
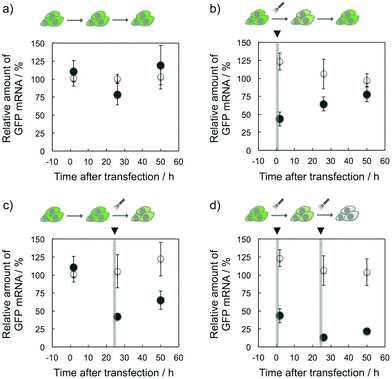
The target selective and photoresponsive gene regulation effect of K-ASs may be a powerful tool in the fields of biotechnology and chemical biology. Finally, we tried to regulate temporally the GFP expression in cells by the photoirradiation with K-AS-a1. As shown in Fig. 5b, the GFP gene expression level was 60% decreased by the photoirradiation at 0 h after the transfection and then the expression level was monotonically increased, suggesting that the expression level was suppressed by the photo-triggered antisense effect and recovered by the spontaneous expression of the GFP gene. On the other hand, in the case of photoirradiation at 24 h after the transfection (Fig. 5c), the suppression was observed only at 26 h after the transfection, suggesting that the temporal regulation of gene expression was achieved by photoirradiation with photoreactive AS-ODNs. Ninety percent suppression of gene expression was observed in the case of photoirradiation performed twice (0 and 24 h) at 26 h after the transfection (Fig. 5d), suggesting that the expression level was temporally controllable by the frequency of the photoirradiation. Surprisingly, the gene silencing effect of K-AS-a1 with the photoirradiation performed twice was comparable with the effect of siRNA treatment (Fig. S5†), suggesting that our photoreactive AS-ODN has potential to be a gene silencer which is better than siRNA with an appropriate backbone modification. As phosphorothioate-modified ODNs are not degraded in cytoplasmic and nuclear extracts of HeLa cells,11 our photoreactive AS-ODNs would remain in the cells throughout the incubation, although the dilution would occur through cell growth. Since the doubling time of the HeLa cells is ca. 24 h, the concentration of photoreactive AS-ODNs in the cells was diluted at least 4-fold after 48 h of incubation. Together with the dose dependence of K-AS-a1 (Fig. 3b), the photoresponsive antisense effect might be decreased largely after 48 h of incubation. Further modification of ODNs that could enhance the binding affinity, such as 2′-MOE and LNA, can be expected for providing long-lasting photoresponsive antisense effect.
In conclusion, we have clearly demonstrated that photoreactive AS-ODNs having CNVK act as effective photo-regulators of constitutive GFP gene expression in living cells with only 10 s of 366 nm irradiation and that the antisense effect is temporally controllable by the photoirradiation. As the irradiation time required is shorter than that in the case of other photoresponsive AS-ODNs reported (it requires some minutes of UV irradiation),12 we believe that our technology is superior for regulating endogenous gene expression in living cells without UV damage. As the time and area of photoirradiation are completely controllable, this method largely contributes to spatiotemporal regulation of endogenous gene expression in cells or bioorgans. Together with the endoscopic drug delivery and localized photoirradiation, our photoreactive AS-ODNs may be a powerful drug for skin, cervical or gastrointestinal cancer treatment.
Notes and references
- (a) P. S. Miller, L. T. Braiterman and P. O. Ts'o, Biochemistry, 1977, 16, 1988 CrossRef CAS; (b) P. C. Zamecnik and M. L. Stephenson, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 280 CrossRef CAS.
- (a) C. Marcus-Sekura, A. M. Woerner, K. Shinozuka, G. Zon and G. V. J. Quinnan, Nucleic Acids Res., 1987, 15, 5749 CrossRef CAS PubMed; (b) C. Cazenave, C. A. Stein, N. T. Loreau, N. Thuong, L. M. Neckers, C. Subasinghe, C. Hélène, J. S. Cohen and J. J. Toulmé, Nucleic Acids Res., 1989, 17, 4255 CrossRef CAS PubMed.
- (a) P. E. Nielsen, M. Egholm, R. H. Berg and O. Buchardt, Science, 1991, 254, 1497 CAS; (b) K. Kaihatsu, K. Huffman and D. R. Corey, Biochemistry, 2004, 43, 14340 CrossRef CAS PubMed; (c) T. Shiraishi and P. E. Nielsen, Bioconjugate Chem., 2012, 23, 196 CrossRef CAS PubMed.
- (a) A. A. Koshikin, S. K. Singh, P. Nielsen, V. K. Rajwanshi, R. Kumar, M. Meldgaard, C. E. Olsen and J. Wengel, Tetrahedron, 1998, 54, 3607 CrossRef; (b) S. Obika, D. Nanbu, Y. Hari, J. Andoh and K. Morio, Tetrahedron Lett., 1998, 39, 5401 CrossRef CAS; (c) S. K. Singh, R. Kumar and J. Wengel, J. Org. Chem., 1998, 63, 10035 CrossRef CAS; (d) R. Kurnar, S. K. Singh, A. A. Koshkin, V. K. Rajwanshi and M. Meldgaard, Bioorg. Med. Chem. Lett., 1998, 8, 2219 CrossRef; (e) C. Wahlestedt, P. Salmi, L. Good, J. Kela, T. Johnsson, T. Hökfelt, C. Broberger, F. Porreca, J. Lai, K. Ren, M. Ossipov, A. Koshkin, N. Jakobsen, J. Skouv, H. Oerum, M. H. Jacobsen and J. Wengel, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 5633 CrossRef CAS.
- (a) B. P. Monia, J. F. Johnston, T. Geiger, M. Muller and D. Fabbro, Nat. Med., 1996, 2, 668 CrossRef CAS PubMed; (b) H. Yin, C. Betts, A. F. Saleh, G. D. Ivanova, H. Lee, Y. Seow, D. Kim, M. J. Gait and M. J. A. Wood, Mol. Ther., 2010, 18, 819 CrossRef CAS PubMed; (c) E. M. Straarup, N. Fisker, M. Hedtjärn, M. W. Lindholm, C. Rosenbohm, V. Aarup, H. F. Hansen, H. Ørum, J. B. R. Hansen and T. Koch, Nucleic Acids Res., 2010, 38, 7100 CrossRef CAS PubMed.
- A. Tallafuss, D. Gibson, P. Morcos, Y. Li, S. Seredick, J. Eisen and P. Washbourne, Development, 2012, 139, 1691 CrossRef CAS PubMed.
- (a) D. Matsunaga, H. Asanuma and M. Komiyama, J. Am. Chem. Soc., 2004, 126, 11452 CrossRef CAS PubMed; (b) H. Asanuma, X. Liang, H. Nishioka, D. Matsunaga, M. Liu and M. Komiyama, Nat. Protocols, 2007, 2, 203 CrossRef CAS PubMed.
- (a) M. Higuchi, A. Yamayoshi, T. Yamaguchi, R. Iwase, T. Yamaoka, A. Kobori and A. Murakami, Nucleosides Nucleotides Nucleic Acids, 2007, 26, 277 CrossRef CAS PubMed; (b) M. Higuchi, A. Kobori, A. Yamayoshi and A. Murakami, Bioorg. Med. Chem., 2009, 17, 475 CrossRef CAS PubMed; (c) M. Higuchi, A. Yamayoshi, K. Kato, A. Kobori, N. Wake and A. Murakami, Oligonucleotides, 2010, 20, 37 CrossRef CAS PubMed.
- (a) Y. Yoshimura and K. Fujimoto, Org. Lett., 2008, 10, 3227 CrossRef CAS PubMed; (b) Y. Yoshimura, T. Ohtake, H. Okada and K. Fujimoto, ChemBioChem, 2009, 10, 1473 CrossRef CAS PubMed; (c) K. Fujimoto, K. Hiratsuka-Konishi, T. Sakamoto and Y. Yoshimura, Chem. Commun., 2010, 40, 7545 RSC; (d) K. Fujimoto, K. Hiratsuka-Konishi, T. Sakamoto and Y. Yoshimura, ChemBioChem, 2010, 11, 1661 CrossRef CAS PubMed; (e) K. Fujimoto, K. Hiratsuka-Konishi, T. Sakamoto, T. Ohtake, K. Shinohara and Y. Yoshimura, Mol. BioSyst., 2012, 8, 491 RSC; (f) A. Shigeno, T. Sakamoto, Y. Yoshimura and K. Fujimoto, Org. Biomol. Chem., 2012, 10, 7820 RSC; (g) K. Fujimoto, S. Kishi and T. Sakamoto, Photochem. Photobiol., 2013, 89, 1095 CrossRef CAS PubMed; (h) K. Fujimoto, K. Konishi-Hiratsuka and T. Sakamoto, Int. J. Mol. Sci., 2013, 14, 5765 CrossRef PubMed; (i) K. Fujimoto, A. Yamada, Y. Yoshimura, T. Tsukaguchi and T. Sakamoto, J. Am. Chem. Soc., 2013, 135, 16161 CrossRef CAS PubMed.
- J. R. Bertrand, M. Pottier, A. Vekris, P. Opolon, A. Maksimenko and C. Malvy, Biochem. Biophys. Res. Commun., 2002, 296, 1000 CrossRef CAS.
- G. D. Hoke, K. Draper, S. M. Freier, C. Gonzalez, V. B. Driver, M. C. Zounes and D. J. Ecker, Nucleic Acids Res., 1991, 19, 5743 CrossRef CAS PubMed.
- (a) A. Deiters, R. A. Garner, H. Lusic, J. M. Govan, M. Dush, N. M. Nascone-Yoder and J. A. Yoder, J. Am. Chem. Soc., 2010, 132, 15644 CrossRef CAS PubMed; (b) J. M. Govan, R. Uprety, M. Thomas, H. Lusic, M. O. Lively and A. Deiters, ACS Chem. Biol., 2013, 8, 2272 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, MALDI-TOF-MS data, melting temperature of AS-ODNs, PAGE analysis of the photo-cross-linking with complementary oligoRNA, expression level of other endogenous genes, and the gene silencing effect of siRNA for GFP. See DOI: 10.1039/c4bm00117f |
| This journal is © The Royal Society of Chemistry 2014 |