Neurons on nanometric topographies: insights into neuronal behaviors in vitro
Mi-Hee
Kim†
a,
Matthew
Park†
a,
Kyungtae
Kang
ab and
Insung S.
Choi
*ab
aCenter for Cell-Encapsulation Research and Molecular-Level Interface Research Center, Department of Chemistry, KAIST, Daejeon 305-701, Korea
bDepartment of Bio and Brain Engineering, KAIST, Daejeon 305-701, Korea. E-mail: ischoi@kaist.ac.kr; Fax: +82-42-350-2810; Tel: +82-42-350-2840
First published on 6th December 2013
Abstract
Topography, the physical characteristics of an environment, is one of the most prominent stimuli neurons can encounter in the body. Many aspects of neurons and neuronal behavior are affected by the size, shape, and pattern of the physical features of the environment. A recent increase in the use of nanometric topographies, due to improved fabrication techniques, has resulted in new findings on neuronal behavior and development. Factors such as neuron adhesion, neurite alignment, and even the rate of neurite formation have all been highlighted through nanotopographies as complex phenomena that are driven by intricate intracellular mechanisms. Nanotopographies are suitable platforms, not only for fundamental studies on neuronal development, but also in practical applications, including multielectrode array devices and neuro-regenerative medicine. We reviewed recent publications that address the effects of nanotopography on neurons and categorized the observed behaviors as adherence, directional guidance, or accelerated outgrowth. We also discussed possible biological mechanisms of the molecular and cellular responses to topography, and suggested future perspectives for this field.
Introduction
Neurons are highly specialized, adherent cells that are pervasive throughout the body. As a result, each neuron can face a drastically different physical environment, from bones to muscles. More importantly, neurons are able to detect the difference in the geometry, roughness, or even rigidity of surfaces, and respond to such physical factors in multiple ways.Interest in the relationship between topography and neuronal development began with the belief that flat coverslips, which were the standard substrate for most neuronal cultures, were poor representations of an in vivo environment. The field continued to progress in an effort to create semi-3D environments in vitro, which would more accurately model the physical features found in vivo. Early topographical experimentation had been limited to substrates with micrometric structures,1,2 and despite the diverse surfaces utilized in these experiments (pillars,3,4 grooves,5–8 and cell-mimetic surfaces9) the prominently observed neuronal response was guided neurite extension along or between the features. As nanofabrication techniques advanced and nanometric structures became more common, additional neuronal responses were observed, such as improved adhesion and accelerated neurite development (Fig. 1). These reports implied that neurons possess the machinery to detect nanometric topographies, and can respond to those minute cues in a nontrivial manner.
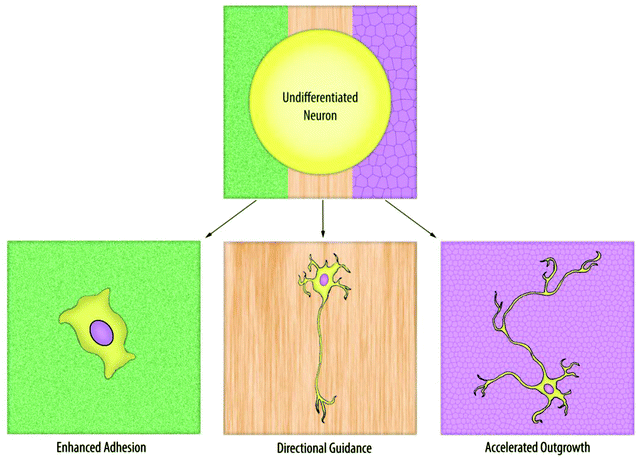 | ||
| Fig. 1 Neurons are sensitive to the physical, subcellular features of a substrate and can exhibit a variety of different morphological and cellular changes in response to different nanotopographies. | ||
More recently, researchers have focused on the intracellular mechanism of neuronal responses. The translation of physical cues is a biologically complex process thought to begin with recognition by membrane receptors as well as physical, cell-to-surface interactions, but the internal biological pathways that follow are still unclear. In this respect, nanotopography would be a more suitable platform on which to study receptor interfaces than microtopography because of the subcellular topographical features that are relevant in scale to the receptor activity. Ultimately, the characterization of this unknown network of pathways will unveil many aspects of the behavior and intracellular processes of neurons, and play an important role in the manipulation of neuronal development for applications in neural circuits, neuroregenerative medicine and prostheses, and much more.
In this article, we reviewed and categorized the different behaviors of neurons on various nanotopographies (Table 1). We also covered reports that tackled the issue of the intracellular pathways involved in nanotopographical recognition and discussed future perspectives for this field.
| Response | Topography | Materials | Neuron types | References |
|---|---|---|---|---|
| Adhesion | Nanoroughness | Si | Substantia nigra | 10, 11 |
| Cortical | 12 | |||
| Hippocampal, cortical | 13 | |||
| TiN | Hippocampal | 14, 15 | ||
| Surface-modified glass | PC12 | 16 | ||
| Au | SH-SY5Y | 17 | ||
| Nanopore | Si | B50 | 18, 19 | |
| SK-N-SH | 21 | |||
| Au | PC12 | 20 | ||
| Pt | PC12 | 22 | ||
| Nanowire | GaP | DRG | 23 | |
| Si, SiGe, Ge, GaN, ZnO | Hippocampal | 25 | ||
| Au | Hippocampal | 26 | ||
| Pt, Si | Cortical | 27, 28 | ||
| Directional guidance and outgrowth | Nanogroove | Polymer (PMMA) | DRG | 29 |
| Polystyrene | PC12 | 30 | ||
| C6 glioma | 38 | |||
| Polymer (COC) | PC12 | 31–35 | ||
| F11 | 36 | |||
| SH-SY5Y, Hippocampal | 37 | |||
| Polymer (PUA) | N1E-155 | 39 | ||
| Polymer (azopolymer) | PC12 | 40 | ||
| Nanofiber | Polymer (PLLA) | DRG | 41, 48 | |
| Primary motor neuron | 42, 43 | |||
| PC12 | 49 | |||
| Polymer (PAN-MA) | DRG | 44 | ||
| Polymer (PCL) | DRG | 45, 47 | ||
| Polymer (polyamide) | Various types | 46 | ||
| Silk fibroin | Various types | 50 | ||
| Polymer (PLCL) | DRG, PC12 | 51 | ||
| Nanowire | GaP | DRG | 24 | |
| Accelerated outgrowth | Nanopore | Si | DRG | 52, 53 |
| AAO | Hippocampal | 61 | ||
| Nanogroove | Si | PC12 | 54 | |
| Nanotube | CNT | Hippocampal | 55–58 | |
| PC12 | 59 | |||
| Nanobead | Si | Hippocampal | 62 |
Adhesion
Adhesion is an easily observable, fundamental indicator of cellular health. In neurons, like other anchorage-dependent cells, both survival and development require proper adhesion to a substrate. As a result, early reports on nanotopography documented the effects of physical surface features on neuronal adhesion. These studies found that, compared with flat surfaces, substrates with a specific range of nanoroughness10–17 or nanoporosity18–22 significantly improved neuronal adhesion and sometimes eliminated the need for neuro-adhesive coatings. Neurons even migrated to areas of “optimum roughness” on these substrates.11Recently, the appearance of more structurally sophisticated nanotopographies has opened avenues for advanced adhesion studies. Nanowires for example, with their high surface area and aspect ratio (length-to-width ratio), are versatile, sub-micrometer units that have become an attractive substrate for neuron cultures.23–26 The first report of neurons cultured on vertical nanowires observed dissociated dorsal root ganglia (DRG) neurons seeded on gallium phosphide (GaP) nanowires.23 The cells adhered to the substrate without a neuro-adhesive coating, and although the scanning electron microscopy (SEM) images revealed penetration of the nanowires into the neurons, viability was not affected. Subsequent studies screened the biocompatibility of other types of nanowires.25
Another example of an adhesion study was the use of silicon nanopillar arrays on multielectrode arrays (MEA) to selectively pin single cortical neurons in predetermined locations.27 Neurons not in contact with the nanopillars migrated freely, but cells that were initially seeded on or eventually encountered the nanopillar arrays were immobilized (Fig. 2a). Transmission electron microscopy (TEM) analysis showed that both cell bodies and neurites interacted more tightly with nanopillars (less than 300 nm in diameter) than with flat substrates.28 The cell-pinning system enabled long-term observation of the electrical activity of an individual neuron, which has been difficult to record for a constantly moving target.27 Nanotopography-based selective adhesion also has many potential uses in other single neuron studies or in microdevices that require the controlled allocation of neurons.
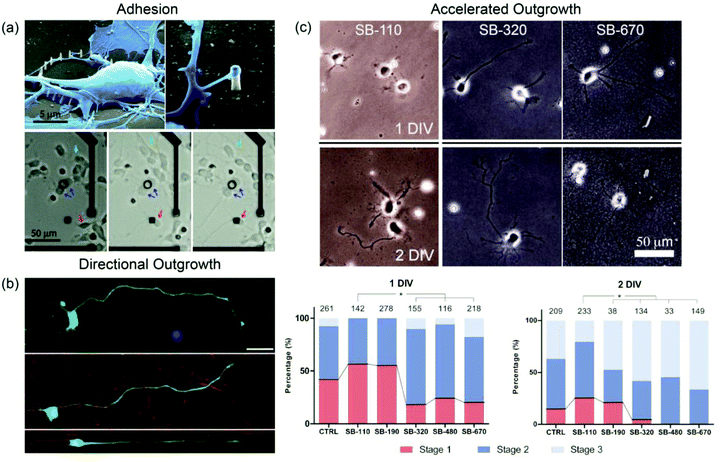 | ||
| Fig. 2 (a) Top. SEM images reveal primary cortical neurons being physically immobilized in a noninvasive manner by silicon nanopillar arrays. Pillars were formed by ion-beam- or e-beam-induced platinum deposition. Bottom. Nanopillar arrays (black circle, black square) were fabricated on an MEA to demonstrate their usefulness in neuron pinning. Green arrows indicate mobile neurons that are unencumbered by nanopillar arrays. Blue arrows indicate immobilized neurons seeded on nanopillar arrays. Red arrows indicate mobile neurons that become immobilized on coming in contact with nanopillar arrays. Reprinted with permission from ref. 27, © 2010, American Chemical Society. (b) Fluorescence images of primary spinal motor neurons cultured on glass (top), randomly oriented PLLA nanofibers (middle), and aligned PLLA nanofibers (bottom). High fidelity to the underlying nanofibers can be observed in neurites developing on the aligned nanofiber substrate. The scale bar is 25 μm. Reprinted with permission from ref. 43, © 2010, John Wiley & Sons, Inc. (c) Phase contrast micrographs of primary hippocampal neurons cultured on silica beads of varying diameters. Beads with diameters greater than 200 nm accelerated stage development and neurite outgrowth in neurons. Beads with diameters less than 200 nm had neurons with developmental rates comparable to neurons grown on glass (DIV = days in vitro, SB-# = silica beads with # nm diameters). Reprinted with permission from ref. 62, © 2012, Wiley-VCH. | ||
Directional guidance and outgrowth
The most distinct morphological feature of neurons is the neurites. These processes branch out to probe the environment, and more importantly, act as carriers of electrical signals. Early topographical studies discovered that neurites could be guided by micrometer-sized grooves and ridges to grow in a single direction, with the fidelity and directionality of neurite guidance being imposed mainly by the physical constraints of the grooves. In recent reports, neurons and neurites have also shown guidance effects on nanometric features, which suggest the presence of intimate interactions between neurons and nanotopography in neurite development.29–40Electrospun nanofiber bundles, particularly, have become popular as substrates for topographical studies.41–45 Nanofibers make excellent scaffolds, especially in neuroregenerative applications, because they can be fabricated with many different biocompatible materials, and can be shaped to form anything between few millimeter bridges to centimeter long patches. Martin et al. reported the first case of primary neurons (DRG explants) cultured on aligned poly-L-lactic acid (PLLA) nanofibers with high, intermediate, or random alignment (the average fiber diameter was about 500 nm).41 Neurites extended radially from the ganglia, but quickly aligned once contact was made with the fibers. Primary motor neurons cultured on PLLA nanofibers displayed the same alignment behavior (Fig. 2b).42,43
With the ability to direct axonal growth being fairly established, focus is shifting to coating or functionalizing nanofibers to improve neuron development.46–51 For example, biochemical stimulators, such as neurotropic factor (GDNF) or nerve growth factor (NGF), have been incorporated into silk fibroin fibers to foster axonal growth.50 Even multi-walled carbon nanotubes (MWCNTs) have been explored as a possible coating material for electrospun nanofibers: MWCNT-coated poly(L-lactic acid-co-caprolactone) (PLCL) fibers were reported to significantly increase neurite outgrowth in DRG explants compared with non-coated fibers.51
Accelerated neurite outgrowth
The length of neurites is a staple measurement in neuron characterization. Many surfaces, ranging from nanoporous silica substrates52,53 and grooves54 to functionalized carbon nanotubes,55–59 are known to induce longer neurites. Some groups, however, regard neurite outgrowth as a more complex phenomenon; rather than measure neurite length at a single point in time, the groups continually observed neurite morphology during the development of the neurons to determine the rates of neurite formation and elongation. Corey et al. cultured primary motor neurons on electrospun PLLA nanofibers and reported that while the number of neurites and the length of major neurites grown on planar PLLA films and PLLA nanofibers were the same, the initial formation of neurites and major neurite determination were more rapid on the latter substrate. The development of the neurons was further analyzed based on the five stages of hippocampal neuron development originally proposed by Banker et al.60 Furthermore, they also observed that the maturation of minor neurites was limited on fibers, either due to the physical restrictions imposed by the fibers or by undefined factors that directed cells towards axonal growth.43Choi et al. have also conducted investigations of accelerated neurite outgrowth. The group cultured hippocampal neurons on three different anodized aluminum oxide (AAO) substrates: small-concave (60 nm in pitch), large-concave (400–450 nm), or nanoporous (400–450 nm).61 Large-concave and nanoporous structures accelerated axonal outgrowth and neuronal polarization, regardless of their depth. In a separate report, silica beads with diameters between 100 nm and 700 nm were used as substrates to demonstrate pitch-dependent acceleration effects.62 Beads with diameters larger than 200 nm induced neuritogenesis in hippocampal neurons much earlier than beads smaller than 200 nm (Fig. 2c). These studies collectively indicate that neurons can distinguish nanotopography and that neuritogenesis is affected by a threshold pitch.
Biophysical mechanism
One of the great challenges that remain in this field is the elucidation of the relationship between nanotopography, neuronal response, and the mechanism by which neurons translate physical cues to biological responses. While neurons clearly possess cellular machinery capable of nanometric recognition, the exact biological components and intracellular pathways involved are still a mystery. A few groups have attempted mechanistic studies in the observation of nanotopographical effects on neurons.Cytoskeletal dynamics are believed to be a central factor in topography-induced responses.63 F-actin in neurites, one of many cytoskeletal components, often assembles into networks in the shape of the underlying topography43 and actively participates in filopodial/lamellapodial protrusion and retraction. For example, impairment of the F-actin polymerization process completely deletes the ability of neurons to distinguish and respond to nanotopographical cues (Fig. 3a).62 Observations of neurite formation on glass coverslips have actually shown that local actin instability in a neurite was a determining factor for its development into an axon.64 It is possible that nanotopography induces local actin instability in an unidentified manner to promote neurite alignment or development at an accelerated pace.
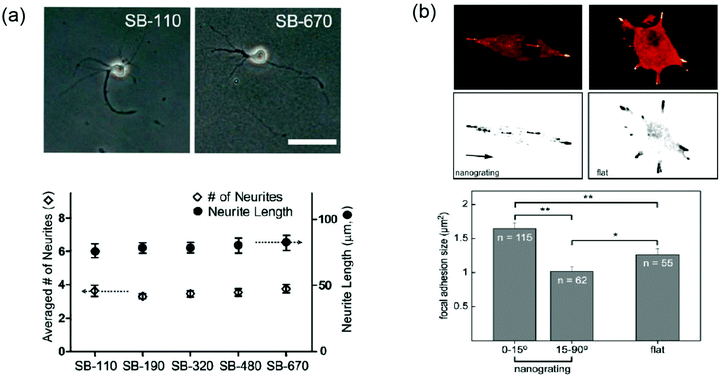 | ||
| Fig. 3 (a) Top. Phase-contrast micrographs of hippocampal neurons cultured on bead-packed substrates (SB-110 and SB-670) and treated with cytochalasin D, an F-actin-depolymerization agent. Bottom. After cytochalasin D treatment, the same phenotype (average number of neurites and neurite length) was observed on all substrates including the glass control. The scale bar is 50 μm. Reprinted with permission from ref. 62, © 2012, Wiley-VCH. (b) Top. Distribution of paxillin-EGFP fluorescent signal (focal adhesion) on nanograting (left) and flat (right) substrates. On nanogratings, aligned FAs were detected at the tips of bipolar cells while several unconstrained FAs were observed in multipolar cells on the flat substrate. Bottom. Statistical comparison of focal adhesion sizes on nanograting and flat substrates. Sizes of aligned FAs (0–15°) were larger than those of misaligned FAs (15–90°) and FAs generated on flat substrates. Reprinted with permission from ref. 33, © 2010, Elsevier. | ||
In addition to the mapping of the intracellular cytoskeletal mechanisms, attempts to characterize the interface between neuron-like cells and nanotopographical surfaces have been made. Neuronal receptor activity is difficult to define not only because of the small scale at which it occurs, but also because receptors can vary greatly between cell types. Beltram et al. have published multiple reports that extensively target the behavior of PC12 cells, a line of neuron-like cells, cultured on cyclic olefin copolymer (COC) nanogrooves, and focused especially on the formation of focal adhesions (FAs).32–34 FAs are the result of smaller adhesion plaques (<1 μm2) consisting of various proteins, which mature into larger contacts that are closely linked to the actin cytoskeleton. While the existence of FAs in neurons is uncertain, the complexes are present in almost all adherent cells, including PC12 cells, and are important factors in the determination of cell shape and morphology.65 Analysis of the FAs in differentiated PC12 cells revealed that FA sizes were significantly larger in neurites aligned along nanogratings than the ones in misaligned neurites or neurites that grew on flat coverslips.33 The angular restriction imposed by the nanogratings was thought to physically constrain the maturation of FAs in misaligned neurites, while FAs in aligned neurites faced no such confinement (Fig. 3b). The correlation between FA size and polarity selection hinted that larger FA complexes and the longer persistence of those complexes might trigger the formation of cytoskeletal actin in those areas. This event prevents the collapse of neurites and facilitates a positive feedback loop, which causes the neurites of PC12 cells to align and persist along the nanogratings.33 The continual collapse of misaligned neurites, on the other hand, prevents the establishment of polarity perpendicular to the grooves.
In order to further understand the role of FAs in neurite alignment, Beltram et al. inhibited components in pathways known to foster the maturation of FAs (ROCK and myosin-II). The inhibition of precursors to FA maturation disrupted alignment along nanogrooves in PC12 cells. The group conducted additional inhibition studies in multiple pathways with varied neurotrophic factors and inhibitors to indirectly affect the formation of FAs.32 Their results demonstrated that FA maturation is a key component of cellular responses to nanotopography and that topography-translating intracellular mechanisms have many complex, interconnecting components.
The current knowledge of the mechanisms by which neurons recognize nanotopographies is far from completion, mostly due to the nascent nature of the field. The complexity of the pathways makes it a daunting task to fully map the network, but continued research on the intracellular mechanisms of neurons is necessary for a comprehensive understanding of nanotopographical effects on neuronal development.
Conclusions
In this review, we categorized neuronal responses on nanotopographies as adhesion, directional guidance, or accelerated outgrowth. Many studies have gone beyond the simple observation of neuron behavior, and have begun using nanotopographies in practical applications, such as the fabrication of nanopillars for selective neuron-pinning arrays, or nanofiber scaffolds to induce the directional regeneration of neurites. Additionally, researchers are implementing nanotopographical substrates as platforms for mechanistic studies with promising results. Small facets of the relationship between topography and neuronal behavior have been revealed through the use of nanotopography, like the possible role of actin dynamics and focal adhesion maturation in the topography-sensing mechanism of neurons. Ultimately, extensive research that details the intracellular pathways of a neuron must occur alongside continued observations of nanotopographical effects for this specific field to fully understand and manipulate neurons.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2012R1A3A2026403).References
- A. M. Rajnicek, S. Britland and C. D. McCaig, J. Cell Sci., 1997, 110, 2905–2913 Search PubMed.
- A. M. Rajnicek and C. D. McCaig, J. Cell Sci., 1997, 110, 2915–2924 CAS.
- N. M. Dowell-Mesfin, M. Abdul-Karim, A. M. P. Turner, S. Schanz, H. G. Craighead, B. Roysam, J. N. Turner and W. Shain, J. Neural Eng., 2004, 1, 78–90 CrossRef CAS PubMed.
- J. N. Hanson, M. J. Motala, M. L. Heien, M. Gillette, J. Sweedler and R. G. Nuzzo, Lab Chip, 2009, 9, 122–131 RSC.
- H. G. Craighead, C. D. James and A. M. P. Turner, Curr. Opin. Solid State Mater. Sci., 2001, 5, 177–184 CrossRef CAS.
- J. S. Goldner, J. M. Bruder, G. Li, D. Gazzola and D. Hoffman-Kim, Biomaterials, 2006, 27, 460–472 CrossRef CAS PubMed.
- N. Gomez, S. Chen and C. E. Schmidt, J. R. Soc., Interface, 2007, 4, 223–233 CrossRef CAS PubMed.
- L. Yao, S. Wang, W. Cui, R. Sherlock, C. O'Connell, G. Damodaran, A. Gorman, A. Windebank and A. Pandit, Acta Biomater., 2009, 5, 580–588 CrossRef CAS PubMed.
- J. M. Bruder, A. P. Lee and D. Hoffman-Kim, J. Biomater. Sci., Polym. Ed., 2007, 18, 967–982 CrossRef CAS PubMed.
- Y. W. Fan, F. Z. Cui, L. N. Chen, Y. Zhai, Q. Y. Xu and I.-S. Lee, Appl. Surf. Sci., 2002, 187, 313–318 CrossRef CAS.
- Y. W. Fan, F. Z. Cui, S. P. Hou, Q. Y. Xu, L. N. Chen and I.-S. Lee, J. Neurosci. Methods, 2002, 120, 17–23 CrossRef CAS.
- S. P. Khan, G. G. Auner and G. M. Newaz, Nanomedicine, 2005, 1, 125–129 CAS.
- J. Ma, B. F. Liu, Q. Y. Xu and F. Z. Cui, Colloids Surf., B, 2005, 44, 152–157 CrossRef CAS PubMed.
- L. A. Cyster, D. M. Grant, K. G. Parker and T. L. Parker, Biomol. Eng., 2002, 19, 171–175 CrossRef CAS.
- L. A. Cyster, K. G. Parker, T. L. Parker and D. M. Grant, Biomaterials, 2004, 25, 97–107 CrossRef CAS.
- G. Lamour, N. Journiac, S. Souès, S. Bonneau, P. Nassoy and A. Hamraoui, Colloids Surf., B, 2009, 72, 208–218 CrossRef CAS PubMed.
- V. Brunetti, G. Maiorano, L. Rizzello, B. Sorce, S. Sabella, R. Cingolani and P. P. Pompa, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6264–6269 CrossRef CAS PubMed.
- S. C. Bayliss, L. D. Buckberry, I. Fletcher and M. J. Tobin, Sens. Actuators, A, 1999, 74, 139–142 CrossRef CAS.
- A. V. Sapelkin, S. C. Bayliss, B. Unal and A. Charalambou, Biomaterials, 2006, 27, 842–846 CrossRef CAS PubMed.
- F. Haq, V. Anandan, C. Keith and G. Zhang, Int. J. Nanomed., 2007, 2, 107–115 CrossRef CAS.
- Y. L. Khung, G. Barritt and N. H. Voelcker, Exp. Cell Res., 2008, 314, 789–800 CrossRef CAS PubMed.
- S. Schlie-Wolter, A. Deiwick, E. Fadeeva, G. Paasche, T. Lenarz and B. N. Chichkov, ACS Appl. Mater. Interfaces, 2013, 5, 1070–1077 CAS.
- W. Hällström, T. Mårtensson, C. Prinz, P. Gustavsson, L. Montelius, L. Samuelson and M. Kanje, Nano Lett., 2007, 7, 2960–2965 CrossRef PubMed.
- C. Prinz, W. Hällström, T. Mårtensson, L. Samuelson, L. Montelius and M. Kanje, Nanotechnology, 2008, 19, 345101–345106 CrossRef PubMed.
- K.-Y. Lee, S. Shim, I.-S. Kim, H. Oh, S. Kim, J.-P. Ahn, S.-H. Park, H. Rhim and H.-J. Choi, Nanoscale Res. Lett., 2010, 5, 410–415 CrossRef CAS PubMed.
- A. Islam and L. Menon, Adv. Mater. Res., 2012, 383–390, 3863–3868 CAS.
- C. Xie, L. Hanson, W. Xie, Z. Lin, B. Cui and Y. Cui, Nano Lett., 2010, 10, 4020–4024 CrossRef CAS PubMed.
- L. Hanson, Z. C. Lin, C. Xie, Y. Cui and B. Cui, Nano Lett., 2012, 12, 5815–5820 CrossRef CAS PubMed.
- F. Johansson, P. Carlberg, N. Danielsen, L. Montelius and M. Kanje, Biomaterials, 2006, 27, 1251–1258 CrossRef CAS PubMed.
- M. Cecchini, G. Bumma, M. Serresi and F. Beltram, Nanotechnology, 2007, 18, 505103–505109 CrossRef.
- A. Ferrari, M. Cecchini, R. Degl'Innocenti and F. Beltram, IEEE Trans. Biomed. Eng., 2009, 56, 2692–2696 CrossRef PubMed.
- A. Ferrari, P. Faraci, M. Cecchini and F. Beltram, Biomaterials, 2010, 31, 2565–2573 CrossRef CAS PubMed.
- A. Ferrari, M. Cecchini, M. Serresi, P. Faraci, D. Pisignano and F. Beltram, Biomaterials, 2010, 31, 4682–4694 CrossRef CAS PubMed.
- A. Ferrari, M. Cecchini, A. Dhawan, S. Micera, I. Tonazzini, R. Stabile, D. Pisignano and F. Beltram, Nano Lett., 2011, 11, 505–511 CrossRef CAS PubMed.
- I. Tonazzini, S. Meucci, P. Faraci, F. Beltram and M. Cecchini, Biomaterials, 2013, 34, 6027–6036 CrossRef CAS PubMed.
- P. Wieringa, I. Tonazzini, S. Micera and M. Cecchini, Nanotechnology, 2012, 23, 275102–275115 CrossRef PubMed.
- I. Tonazzini, A. Cecchini, Y. Elgersma and M. Cecchini, Adv. Healthcare Mater., 2013 DOI:10.1002/adhm.201300216.
- B. Zhu, Q. Zhang, Q. Lu, Y. Xu, J. Yin, J. Hu and Z. Wang, Biomaterials, 2004, 25, 4215–4223 CrossRef CAS PubMed.
- K.-J. Jang, M. S. Kim, D. Feltrin, N. L. Jeon, K.-Y. Suh and O. Pertz, PLoS One, 2010, 5, e15966 CAS.
- R. Barillé, R. Janik, S. Kucharski, J. Eyer and F. Letournel, Colloids Surf., B, 2011, 88, 63–71 CrossRef PubMed.
- J. M. Corey, D. Y. Lin, K. B. Mycek, Q. Chen, S. Samuel, E. L. Feldman and D. C. Martin, J. Biomed. Mater. Res., Part A, 2007, 83, 636–645 CrossRef PubMed.
- J. M. Corey, C. C. Gertz, B.-S. Wang, L. K. Birrell, S. L. Johnson, D. C. Martin and E. L. Feldman, Acta Biomater., 2008, 4, 863–875 CrossRef CAS PubMed.
- C. C. Gertz, M. K. Leach, L. K. Birrell, D. C. Martin, E. L. Feldman and J. M. Corey, Dev. Neurobiol., 2010, 70, 589–603 CrossRef CAS PubMed.
- Y.-T. Kim, V. K. Haftel, S. Kumar and R. V. Bellamkonda, Biomaterials, 2008, 29, 3117–3127 CrossRef CAS PubMed.
- J. Xie, M. R. MacEwan, X. Li, S. E. Sakiyama-Elbert and Y. Xia, ACS Nano, 2009, 3, 1151–1159 CrossRef CAS PubMed.
- I. Ahmed, H.-Y. Liu, P. C. Mamiya, A. S. Ponery, A. N. Babu, T. Weik, M. Schindler and S. Meiners, J. Biomed. Mater. Res., Part A, 2006, 76, 851–860 CrossRef PubMed.
- E. Schnell, K. Klinkhammer, S. Balzer, G. Brook, D. Klee, P. Dalton and J. Mey, Biomaterials, 2007, 28, 3012–3025 CrossRef CAS PubMed.
- S. Patel, K. Kurpinski, R. Quigley, H. Gao, B. S. Hsiao, M.-M. Poo and S. Li, Nano Lett., 2007, 7, 2122–2128 CrossRef CAS PubMed.
- H. S. Koh, T. Yong, C. K. Chan and S. Ramakrishna, Biomaterials, 2008, 29, 3574–3582 CrossRef CAS PubMed.
- S. Madduri, M. Papaloïzos and B. Gander, Biomaterials, 2010, 31, 2323–2334 CrossRef CAS PubMed.
- G.-Z. Jin, M. Kim, U. S. Shin and H.-W. Kim, Neurosci. Lett., 2011, 501, 10–14 CrossRef CAS PubMed.
- F. Johansson, M. Kanje, C. Eriksson and L. Wallman, Phys. Status Solidi, 2005, 2, 3258–3262 CrossRef CAS.
- F. Johansson, M. Kanje, C. E. Linsmeier and L. Wallman, IEEE Trans. Biomed. Eng., 2008, 55, 1447–1449 CrossRef PubMed.
- J. D. Foley, E. W. Grunwald, P. F. Nealey and C. J. Murphy, Biomaterials, 2005, 26, 3639–3644 CrossRef CAS PubMed.
- M. P. Mattson, R. C. Haddon and A. M. Rao, J. Mol. Neurosci., 2000, 14, 175–182 CrossRef CAS.
- H. Hu, Y. Ni, V. Montana, R. C. Haddon and V. Parpura, Nano Lett., 2004, 4, 507–511 CrossRef CAS PubMed.
- H. Hu, Y. Ni, S. K. Mandal, V. Montana, B. Zhao, R. C. Haddon and V. Parpura, J. Phys. Chem. B, 2005, 109, 4285–4289 CrossRef CAS PubMed.
- E. B. Malarkey, K. A. Fisher, E. Bekyarova, W. Liu, R. C. Haddon and V. Parpura, Nano Lett., 2009, 9, 264–268 CrossRef CAS PubMed.
- S. Agarwal, X. Zhou, F. Ye, Q. He, G. C. K. Chen, J. Soo, F. Boey, H. Zhang and P. Chen, Langmuir, 2010, 26, 2244–2247 CrossRef CAS PubMed.
- C. G. Dotti, C. A. Sullivan and G. A. Banker, J. Neurosci., 1988, 8, 1454–1468 CAS.
- W. K. Cho, K. Kang, G. Kang, M. J. Jang, Y. Nam and I. S. Choi, Angew. Chem., Int. Ed., 2010, 49, 10114–10118 CAS.
- K. Kang, S.-E. Choi, H. S. Jang, W. K. Cho, Y. Nam, I. S. Choi and J. S. Lee, Angew. Chem., Int. Ed., 2012, 51, 2855–2858 CrossRef CAS PubMed.
- J. S. Da Silva and C. G. Dotti, Nat. Rev. Neurosci., 2002, 3, 694–704 CrossRef CAS PubMed.
- F. Bradke and C. G. Dotti, Science, 1999, 283, 1931–1934 CrossRef CAS.
- S. K. Mitra, D. A. Hanson and D. D. Schlaepfer, Nat. Rev. Mol. Cell Biol., 2005, 6, 56–68 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |




