A polymer additive boosts the anti-cancer efficacy of supramolecular nanofibers of taxol†
Chengbiao
Yang‡
,
Meijie
Bian‡
and
Zhimou
Yang
*
State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071, P. R. China. E-mail: yangzm@nankai.edu.cn
First published on 12th December 2013
Abstract
A polymer additive of hyaluronic acid (HA) could boost the anti-cancer efficacy of supramolecular nanofibers of a prodrug of taxol (succinated taxol).
Supramolecular nanofibers of peptides1–7 have shown promising applications in regenerative medicine,8–10 immune boosting,9,11,12 cell culture,13–16 cancer cell inhibition,17–19 drug delivery,20–25 and enzyme detection.26–29 In the field of drug delivery, they can be applied to deliver therapeutic agents either serving as physical carriers30–33 or as self-delivery systems.34–38 Recent research efforts have focused on the development of supramolecular nanofibers of drug–peptide conjugates as self-delivery systems, because they have high and designable drug loadings, show little or no burst release, and exhibit rapid stimuli responsiveness. Several kinds of drugs such as anti-inflammatory and anti-cancer ones have been chosen to conjugate with peptides through hydrolyzable bonds,35,39–41 resulting in supramolecular nanofibers that can constantly release drug molecules over a long period of time. For instance, anti-inflammatory drugs olsalazine36 and dexamethasone42,43 have been connected with peptides via the acid-liable hydrazone bond. Anti-cancer drugs taxol44–46 and camptothecin34,43 have also been conjugated with peptides through ester bonds or disulfide bonds. These self-delivery systems have exhibited high efficacy in modulating the inflammatory response or inhibiting cancer cells.
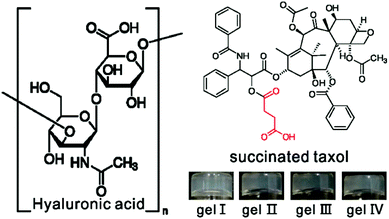
Our group focuses on the development of supramolecular nanofibers of hydrophobic therapeutic agents and we have reported on nanofibers of taxol,45 rapamycin,42 dexamethasone,42,43 and 10-hydroxy camptothecin.43 These nanofibers could release original drug molecules through ester bond hydrolysis. During the synthesis of succinated taxol with a carboxylic acid group for further conjugation with peptides, we occasionally found that succinated taxol itself could form hydrogels upon sonication (Fig. 1). This was the simplest gelator system based on taxol. McNeil's and Adams’ groups had demonstrated that polymer additives have strong effects on the properties of molecular hydrogels.47,48 For example, the addition of dextran could tune the gelation time and mechanical properties of gels of a dipeptide-based gelator.48 Poly(acrylic acid) could dramatically reduce the critical gelation concentration and improve the mechanical properties of gels of pyridine-based gelators.47 We therefore opted to test the effects of a polymer additive of hyaluronic acid (HA in Fig. 1, MW of 770 kDa) on the properties of gels of succinated taxol. HA was a biocompatible macromolecule that had been widely applied in cosmetics, surgery, and regenerative medicine.49–51 We thought that addition of HA might adjust the mechanical properties, morphology of self-assembled nanostructures, and the release behavior of the gels.
We found that a phosphate buffer saline (PBS, pH = 7.4) suspension of succinated taxol could change into a gel upon sonication within one minute (gel I in Fig. 1). The LC-MS result indicated that succinated taxol remained intact in the gel and there were no hydrolysis products (Fig. S-1†) even for up to one month, indicating a different gelation mechanism compared to our previously reported taxol hydrogel formed by ester bond hydrolysis.37 Sonication-induced hydrogelations had been reported by Yi and other groups,52–54 and this was another example of hydrogels formed by this mechanism. The minimum gelation concentration of succinated taxol was about 0.4 wt%. The hydrogels could also form in the presence of HA. As shown in Fig. 1, suspensions of succinated taxol (1.0 wt%, 10 mg mL−1) could form gels upon sonication in the presence of 1, 3, and 6 mg mL−1 of HA (gel II, gel III, and gel IV, respectively). The gel was intact after being soaked with the DMEM containing 10% FBS for 48 h (Fig. S-3†), indicating its good stability in the presence of serum protein.
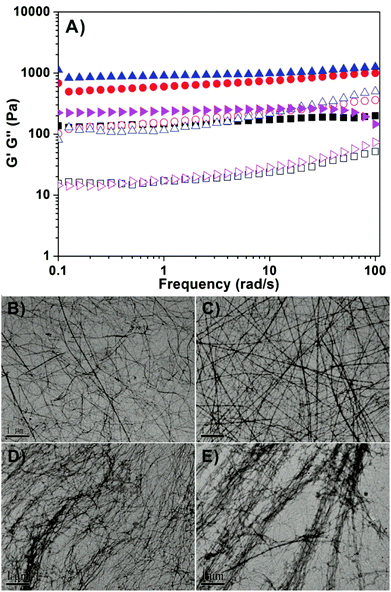
We then characterized the mechanical properties of hydrogels by rheology. As shown in Fig. 2A, we found that both the storage modulus (elasticity or G′) and the loss modulus (viscosity or G′′) exhibited weak frequency dependences between 0.1 and 100 rad s−1. For each gel, the value of G′ was bigger than that of G′′, suggesting the presence of an elastic three dimensional network in the gel. The results also showed that G′ values of gel III and gel IV with more than 3 mg mL−1 of HA were obviously bigger than those of gel I (no HA) and gel II (1 mg mL−1 of HA).55 These observations suggested that the addition of more than 30 wt% of HA could improve the mechanical properties of gels of succinated taxol. We then characterized the nanostructures in the hydrogels by transmission electron microscopy (TEM, Fig. 1B–1E). We observed filamentous structures in all gels and the diameter of fibrils was similar (about 20–25 nm). All fibrils were longer than 10 μm and they entangled with each other to form networks for hydrogelations. It was obviously observed that fibrils in gel III and gel IV formed bundles, while those in both gel I and gel II existed mostly in single form. The presence of bundles of fibrils in gel III and gel IV accounted for their relatively bigger G′ values.
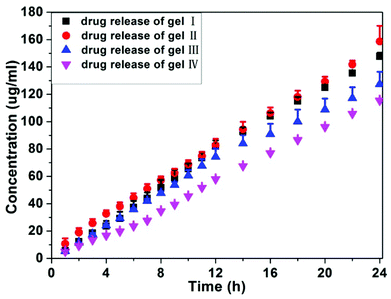
We also monitored the release profile of anti-cancer drugs from the gels at 37 °C (Fig. 3). All hydrogels released succinated taxol during the 24 h experimental time and no taxol was released from gels. Both gel I and gel II exhibited similar release behaviors and there were about 1.48 and 1.58% of succinated taxol being released from gel I and gel II over 24 hours, respectively. Gel III and gel IV with more than 3 mg mL−1 of HA possessed slightly slower release profiles, and there were about 1.27 and 1.15% of succinated taxol being released from gel III and gel IV over 24 hours, respectively. There were no burst releases for four gels, suggesting its good potential for long term release of anti-cancer drugs for cancer therapy. These observations, in combination with results by rheology and TEM, indicated that the addition of more than 3 mg mL−1 of HA to gels of succinated taxol (10 mg mL−1) could slightly modify the mechanical property, morphology of self-assembling nanostructures, and release behavior of the gels.
The anti-tumor efficacy of our hydrogels in the mice tumor model (4T1-luciferase breast tumors in mammary fat pad of female mice) was also evaluated in vivo. We chose gel I and gel III for evaluations. When the volume of breast tumors reached about 30 mm3, we injected the same dosages (10 mg kg−1 of taxol × 4 every other day) of different formulations of taxol into the mice through caudal vein. As shown in Fig. 4, gel I exhibited similar anti-tumor growth efficacy to Taxol®. Mice administrated with Taxol® showed a slight body weight loss during the experimental time (Fig. S-2†), probably due to the presence of organic solvents in Taxol®. Surprisingly, gel III with 30% of HA showed an enhanced anti-tumor growth capacity over gel I and Taxol®. The final volume of tumors was about 3401%, 2278%, 2201%, and 1558% bigger than the original volume of tumors for the PBS control group, Taxol®, gel I, and gel III, respectively. High mass-molecular hyaluronan (6000–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa) could mediate the cancer resistance.56 However, the mass-molecular of HA used in this paper was 770 kDa. Therefore, the better anti-cancer efficacy of nanofibers of succinated taxol in the presence of HA was probably due to the tumor targeting effect, because HA was a ligand for CD44 that was over-expressed in cancer cells.57 There were no obvious body weight losses in groups of mice administrated with our hydrogels (Fig. S-2†), compared to the control group of mice without any treatment. These results suggested promising potential of our hydrogel for cancer therapy. The results also indicated that polymer additives could not only improve the mechanical property of molecular hydrogels, but also boost anti-cancer efficacy of our hydrogels of succinated taxol.
000 kDa) could mediate the cancer resistance.56 However, the mass-molecular of HA used in this paper was 770 kDa. Therefore, the better anti-cancer efficacy of nanofibers of succinated taxol in the presence of HA was probably due to the tumor targeting effect, because HA was a ligand for CD44 that was over-expressed in cancer cells.57 There were no obvious body weight losses in groups of mice administrated with our hydrogels (Fig. S-2†), compared to the control group of mice without any treatment. These results suggested promising potential of our hydrogel for cancer therapy. The results also indicated that polymer additives could not only improve the mechanical property of molecular hydrogels, but also boost anti-cancer efficacy of our hydrogels of succinated taxol.
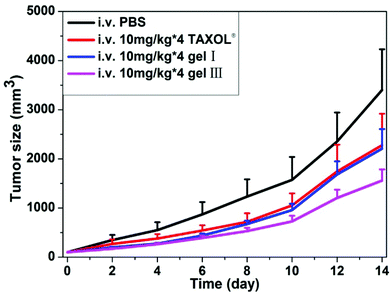 | ||
| Fig. 4 Gel 1 and gel 2 inhibit xenografted mouse breast tumor (4T1-luciferase) growth in vivo (gels were administrated into the caudal vein after tumor sizes reaching ∼30 mm3, n = 8). | ||
In summary, we have reported on a molecular hydrogel of a prodrug of taxol, succinated taxol. The drug loadings in the self-assembling nanofibers of gel I, gel II, gel III, and gel IV were 89.5, 81.4, 68.8, and 55.9 wt%, respectively. The drug loading in nanofibers of gel I was the highest reported up to now. We also found that the addition of a polymer additive HA could slightly enhance the mechanical property of the gel. Moreover, the addition of more than 30 wt% of HA could boost the anti-cancer efficacy of the nanofibers of succinated taxol. We believed that self-assembling nanofibers in our study could also serve as carriers to co-deliver other hydrophobic anti-cancer drugs. Besides, we envision that polymer additives may also improve the anti-cancer efficacy of other self-assembling nanofibers of therapeutic agents.
Notes and references
- Y. Gao, F. Zhao, Q. Wang, Y. Zhang and B. Xu, Chem. Soc. Rev., 2010, 39, 3425–3433 RSC.
- E. K. Johnson, D. J. Adams and P. J. Cameron, J. Mater. Chem., 2011, 21, 2024–2027 RSC.
- J. Raeburn, A. Zamith Cardoso and D. J. Adams, Chem. Soc. Rev., 2013, 42, 5143–5156 RSC.
- J. W. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC.
- R. V. Ulijn and A. M. Smith, Chem. Soc. Rev., 2008, 37, 664–675 RSC.
- Y. Li, Y. Ding, M. Qin, Y. Cao and W. Wang, Chem. Commun., 2013, 49, 8653–8655 RSC.
- M. Zelzer, S. J. Todd, A. R. Hirst, T. O. McDonald and R. V. Ulijn, Biomater. Sci., 2013, 1, 11–39 RSC.
- J. S. Rudra, T. Sun, K. C. Bird, M. D. Daniels, J. Z. Gasiorowski, A. S. Chong and J. H. Collier, ACS Nano, 2012, 6, 1557–1564 CrossRef CAS PubMed.
- J. S. Rudra, Y. F. Tian, J. P. Jung and J. H. Collier, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 622–627 CrossRef CAS PubMed.
- R. N. Shah, N. A. Shah, M. M. Del Rosario Lim, C. Hsieh, G. Nuber and S. I. Stupp, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 3293–3298 CrossRef CAS PubMed.
- J. Chen, R. R. Pompano, F. W. Santiago, L. Maillat, R. Sciammas, T. Sun, H. Han, D. J. Topham, A. S. Chong and J. H. Collier, Biomaterials, 2013, 34, 8776–8785 CrossRef CAS PubMed.
- J. S. Rudra, S. Mishra, A. S. Chong, R. A. Mitchell, E. H. Nardin, V. Nussenzweig and J. H. Collier, Biomaterials, 2012, 33, 6476–6484 CrossRef CAS PubMed.
- V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611–614 CrossRef CAS.
- A. Mahler, M. Reches, M. Rechter, S. Cohen and E. Gazit, Adv. Mater., 2006, 18, 1365–1370 CrossRef CAS.
- Y. F. Tian, J. M. Devgun and J. H. Collier, Soft Matter, 2011, 7, 6005–6011 RSC.
- C. Yang, D. Li, Z. Liu, G. Hong, J. Zhang, D. Kong and Z. Yang, J. Phys. Chem. B, 2011, 116, 633–638 CrossRef PubMed.
- M. C. Branco, D. M. Sigano and J. P. Schneider, Curr. Opin. Chem. Biol., 2011, 15, 427–434 CrossRef CAS PubMed.
- S. M. Standley, D. J. Toft, H. Cheng, S. Soukasene, J. Chen, S. M. Raja, V. Band, H. Band, V. L. Cryns and S. I. Stupp, Cancer Res., 2010, 70, 3020–3026 CrossRef CAS PubMed.
- R. H. Zha, S. Sur and S. I. Stupp, Adv. Healthcare Mater., 2013, 2, 126–133 CrossRef CAS PubMed.
- P.-F. Caponi and R. V. Ulijn, RSC, 2013, 1, 232–255 Search PubMed.
- C. S. Chen, X. D. Xu, Y. Wang, J. Yang, H. Z. Jia, H. Cheng, C. C. Chu, R. X. Zhuo and X. Z. Zhang, Small, 2013, 9, 920–926 CrossRef CAS PubMed.
- J. X. Chen, X. D. Xu, S. Yang, J. Yang, R. X. Zhuo and X. Z. Zhang, Macromol. Biosci., 2013, 13, 84–92 CrossRef CAS PubMed.
- L. Chen, J. Wu, L. Yuwen, T. Shu, M. Xu, M. Zhang and T. Yi, Langmuir, 2009, 25, 8434–8438 CrossRef CAS PubMed.
- W. Xun, D. Q. Wu, Z. Y. Li, H. Y. Wang, F. W. Huang, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Macromol. Biosci., 2009, 9, 1219–1226 CrossRef CAS PubMed.
- F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883–891 RSC.
- J. Chen, W. Wu and A. J. McNeil, Chem. Commun., 2012, 48, 7310–7312 RSC.
- Y. Gao, J. Shi, D. Yuan and B. Xu, Nat Commun, 2012, 3, 1033 CrossRef PubMed.
- M. Ikeda, K. Fukuda, T. Tanida, T. Yoshii and I. Hamachi, Chem. Commun., 2012, 48, 2716–2718 RSC.
- L. L. Lock, A. G. Cheetham, P. Zhang and H. Cui, ACS Nano, 2013, 7, 4924–4932 CrossRef CAS PubMed.
- A. Altunbas, S. J. Lee, S. A. Rajasekaran, J. P. Schneider and D. J. Pochan, Biomaterials, 2011, 32, 5906–5914 CrossRef CAS PubMed.
- K. C. Koehler, D. L. Alge, K. S. Anseth and C. N. Bowman, Biomaterials, 2013, 34, 4150–4158 CrossRef CAS PubMed.
- J. Nanda, A. Biswas and A. Banerjee, Soft Matter, 2013, 9, 4198–4208 RSC.
- S. Soukasene, D. J. Toft, T. J. Moyer, H. Lu, H.-K. Lee, S. M. Standley, V. L. Cryns and S. I. Stupp, ACS Nano, 2011, 5, 9113–9121 CrossRef CAS PubMed.
- A. G. Cheetham, P. Zhang, Y.-a. Lin, L. L. Lock and H. Cui, J. Am. Chem. Soc., 2013, 135, 2907–2910 CrossRef CAS PubMed.
- J. Li, Y. Kuang, Y. Gao, X. Du, J. Shi and B. Xu, J. Am. Chem. Soc., 2012, 135, 542–545 CrossRef PubMed.
- X. Li, J. Li, Y. Gao, Y. Kuang, J. Shi and B. Xu, J. Am. Chem. Soc., 2010, 132, 17707–17709 CrossRef CAS PubMed.
- H. Wang, J. Wei, C. Yang, H. Zhao, D. Li, Z. Yin and Z. Yang, Biomaterials, 2012, 33, 5848–5853 CrossRef CAS PubMed.
- H. Wang and Z. Yang, Soft Matter, 2012, 8, 2344–2347 RSC.
- Y. Gao, Y. Kuang, Z.-F. Guo, Z. Guo, I. J. Krauss and B. Xu, J. Am. Chem. Soc., 2009, 131, 13576–13577 CrossRef CAS PubMed.
- H. Wang, C. Yang, L. Wang, D. Kong, Y. Zhang and Z. Yang, Chem. Commun., 2011, 47, 4439–4441 RSC.
- M. J. Webber, J. B. Matson, V. K. Tamboli and S. I. Stupp, Biomaterials, 2012, 33, 6823–6832 CrossRef CAS PubMed.
- X. Li, C. Yang, Z. Zhang, Z. Wu, Y. Deng, G. Liang, Z. Yang and H. Chen, J. Mater. Chem., 2012, 22, 21838–21840 RSC.
- L. Mao, H. Wang, M. Tan, L. Ou, D. Kong and Z. Yang, Chem. Commun., 2012, 48, 395–397 RSC.
- R. Lin, A. G. Cheetham, P. Zhang, Y.-a. Lin and H. Cui, Chem. Commun., 2013, 49, 4968–4970 RSC.
- C. Yang, D. Li, Q. FengZhao, L. Wang, L. Wang and Z. Yang, Org. Biomol. Chem., 2013, 11, 6946–6951 CAS.
- P. Zhang, A. G. Cheetham, Y.-a. Lin and H. Cui, ACS Nano, 2013, 7, 5965–5977 CrossRef CAS PubMed.
- Y. J. Adhia, T. H. Schloemer, M. T. Perez and A. J. McNeil, Soft Matter, 2012, 8, 430–434 RSC.
- G. Pont, L. Chen, D. G. Spiller and D. J. Adams, Soft Matter, 2012, 8, 7797–7802 RSC.
- M. B. Brown and S. A. Jones, J. Eur. Acad. Dermatol., 2005, 19, 308–318 CrossRef CAS PubMed.
- J. H. Collier, J. P. Camp, T. W. Hudson and C. E. Schmidt, J. Biomed. Mater. Res., 2000, 50, 574–584 CrossRef CAS.
- H. Tan, C. M. Ramirez, N. Miljkovic, H. Li, J. P. Rubin and K. G. Marra, Biomaterials, 2009, 30, 6844–6853 CrossRef CAS PubMed.
- L. Meng, K. Liu, S. Mo, Y. Mao and T. Yi, Org. Biomol. Chem., 2013, 11, 1525–1532 CAS.
- X. Yu, Q. Liu, J. Wu, M. Zhang, X. Cao, S. Zhang, Q. Wang, L. Chen and T. Yi, Chem.–Eur. J., 2010, 16, 9099–9106 CrossRef CAS PubMed.
- M. Zhang, L. Meng, X. Cao, M. Jiang and T. Yi, Soft Matter, 2012, 8, 4494–4498 RSC.
- J. Nanda, A. Biswas, B. Adhikari and A. Banerjee, Angew. Chem., Int. Ed., 2013, 125, 5145–5149 CrossRef.
- X. Tian, J. Azpurua, C. Hine, A. Vaidya, M. Myakishev-Rempel, J. Ablaeva, Z. Mao, E. Nevo, V. Gorbunova and A. Seluanov, Nature, 2013, 499, 346–349 CrossRef CAS PubMed.
- K. Y. Choi, K. H. Min, J. H. Na, K. Choi, K. Kim, J. H. Park, I. C. Kwon and S. Y. Jeong, J. Mater. Chem., 2009, 19, 4102–4107 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Optical image of a gel, LC-MS traces, rheology, preparation of succinated taxol and hydrogel, and details experimental procedure. See DOI: 10.1039/c3bm60252d |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |