Colorimetric screening of β-glucosidase inhibition based on gold nanocomposites†
Cui
Lai
ab,
Guang-Ming
Zeng
*ab,
Dan-Lian
Huang
*ab,
Mei-Hua
Zhao
ab,
Ming
Chen
ab,
Zhen
Wei
ab,
Chao
Huang
ab,
Piao
Xu
ab,
Ning-Jie
Li
ab,
Xue
Li
c and
Chen
Zhang
ab
aCollege of Environmental Science and Engineering, Hunan University, Changsha 410082, Hunan, PR China. E-mail: zgming@hnu.edu.cn; huangdanlian1981@163.com; Fax: +86-731-88823701; Tel: +86-731-88822754
bKey Laboratory of Environmental Biology and Pollution Control, Hunan University, Ministry of Education, Changsha 410082, Hunan, PR China
cDepartment of Bioengineering and Environmental Science, Changsha University, Changsha 410003, Hunan, PR China
First published on 25th November 2013
Abstract
This article presents a simple gold–cellobiose nanocomposites based colorimetric assay for the screening of β-glucosidase inhibitors. The nanocomposites are composed of gold nanoparticles (AuNPs), particular cellobiose substrates, and 6-mercapto-1-hexanol. After β-glucosidase digestion, the AuNPs become more exposed and the attractive force between AuNPs is increased by the modified 6-mercapto-1-hexanol. Consequently, the aggregation of nanocomposites and the red shift of surface plasmon absorption can be observed. The absorbance ratio at 650 nm and 520 nm (A650/A520) of nanocomposites can be used to estimate the β-glucosidase activity. This technology could serve an alternative platform for the efficient screening of β-glucosidase inhibitors. Both the inhibition effect of heavy metals and surfactants on β-glucosidase could be analyzed by the detection of β-glucosidase activity. To summarize, the goal of this technical note is to develop a simple colorimetric method for the screening of β-glucosidase inhibitors.
1. Introduction
The hydrolysis of cellulose depends on cellulase, which usually contains three components: β-glucosidase (EC 3.2.1.21), β-1,4-endoglucanase (EC 3.2.1.4) and β-1,4-exoglucanase (EC 3.2.1.91). Low activity of β-glucosidase may restrict the conversion of cellobiose, which is an inhibitor of both β-1,4-endoglucanase and β-1,4-exoglucanase.1,2 Accordingly, β-glucosidase plays an important role in the hydrolysis of cellulose,3 and significant efforts have been made to improve the application of β-glucosidase.4,5 When planning the application of β-glucosidase, it is very important to consider the environmental stresses affecting the enzymatic activity. The presence of heavy metals has been found to exert a strong effect on the activity of β-glucosidase, and surfactants can influence the enzymatic hydrolysis of cellulose.6–9 Therefore, the study of the influence of such parameters on the enzymatic activity of β-glucosidase, and similarly, development of specific methods for the screening of β-glucosidase inhibitors, is of great importance. A widely used method is recommended by the International Union of Pure and Applied Chemistry (IUPAC), in which β-glucosidase activity is measured from the concentration of enzyme required to produce a certain amount of glucose per min.1,10 The amount of produced glucose is typically determined by a dinitrosalicylic acid (DNS) spectrophotometric determination method. However, the chemicals involved in the preparation of DNS reagents are toxic, and this method demands very careful sampling.11 Many other methods based on the measurement of reducing groups, chromophore or fluorescent group release have also been developed to identify β-glucosidase inhibitors.10 A simple method for the screening of β-glucosidase inhibitors is still necessary since most of the current methods require sophisticated sample preparation and instrument operation.12Recently, polysaccharides have received much attention in bio-nanotechnology because of their special structural characteristics and biodegradability. Considerable studies have shown that polysaccharides can be extensively exploited in the synthesis of nanomaterials.13–16 Herein, the authors present a simple and specific assay based on gold–cellobiose nanocomposites, which enables the determination of β-glucosidase activity in the absence and presence of inhibitors. It could serve as an alternative platform for the efficient screening of β-glucosidase inhibitors. In this method, cellobiose is functionalized onto the gold nanoparticles (AuNPs) as a stabilizing agent and substrate of β-glucosidase, which can recognize the specific structure of cellobiose. After enzymatic degradation, the nanocomposites become more exposed, and the stability of nanocomposites is disrupted. The surface plasmon resonance (SPR) properties of nanocomposites correspondingly change. Therefore, the absorbance ratio at 650 nm and 520 nm (A650/A520) of nanocomposites can be used as a colorimetric sensor for the screening of β-glucosidase inhibitors. This detection method has advantages of ease of operation and short assay time. It also shows satisfactory sensitivity and selectivity. It is believed that the proposed method can provide an alternative tool for the screening of potential β-glucosidase inhibitors.
2. Materials and methods
Enzymes were purchased from Sigma-Aldrich. All the other chemicals were of analytical grade or the highest purity commercially available. Ultrapure water (18.2 MΩ) was obtained from a Milli-Q purification system and used throughout the experiments. Firstly, the nanocomposites were prepared. In a typical experiment, we mixed a diluted solution of cellobiose (10 mL, 0.1%) with an aqueous solution of freshly prepared HAuCl4 (1%). Sodium borohydride (0.5 M) was rapidly added to the mixture solution after 2 h of continuous stirring (cellobiose/HAuCl4/sodium borohydride = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The mixture was left continuously stirring, and the color of solution rapidly changed from pale yellow to deep red. After two centrifuge/wash cycles (15
1, v/v). The mixture was left continuously stirring, and the color of solution rapidly changed from pale yellow to deep red. After two centrifuge/wash cycles (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 4 °C for 20 min), the supernatant solution was discarded and the pellet was resuspended in a total of 5 mL of pH 5.0 citrate–phosphate buffer. It has been reported that polysaccharides can be grafted onto the surfaces of AuNPs.14 In this method, the binding of cellobiose onto AuNPs is probably a result of electrostatic interactions between the electropositive transition metal cations and the ether, hydroxyl groups of cellobiose.17,18 For the preparation of 6-mercapto-1-hexanol (MCH) modified nanocomposites, 0.01 mL MCH (1 mM) was initially added to the freshly prepared nanocomposites solution (10 mL), and incubated at room temperature for 1 h. Then the mixture was centrifuged at 15
000 rpm, 4 °C for 20 min), the supernatant solution was discarded and the pellet was resuspended in a total of 5 mL of pH 5.0 citrate–phosphate buffer. It has been reported that polysaccharides can be grafted onto the surfaces of AuNPs.14 In this method, the binding of cellobiose onto AuNPs is probably a result of electrostatic interactions between the electropositive transition metal cations and the ether, hydroxyl groups of cellobiose.17,18 For the preparation of 6-mercapto-1-hexanol (MCH) modified nanocomposites, 0.01 mL MCH (1 mM) was initially added to the freshly prepared nanocomposites solution (10 mL), and incubated at room temperature for 1 h. Then the mixture was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 4 °C for 20 min and the pellet was resuspended in 5 mL of pH 5.0 citrate–phosphate buffer. The MCH connects to AuNPs through the thiol group, and the hydroxyl group of MCH is exposed on AuNPs. With the consumption of cellobiose, the AuNPs lose stabilizer and the hydroxyl group of MCH may increase the attraction force of AuNPs.
000 rpm, 4 °C for 20 min and the pellet was resuspended in 5 mL of pH 5.0 citrate–phosphate buffer. The MCH connects to AuNPs through the thiol group, and the hydroxyl group of MCH is exposed on AuNPs. With the consumption of cellobiose, the AuNPs lose stabilizer and the hydroxyl group of MCH may increase the attraction force of AuNPs.
For the β-glucosidase activity assay, 0.1 mL of different concentrations of β-glucosidase solution (citrate–phosphate buffer, pH 5.0) was added to 2.0 mL of nanocomposites solution. The solution was incubated at 30 °C because this condition is suitable for the catalysis reaction of β-glucosidase. The nanocomposites solution gradually turned purple with increasing β-glucosidase concentration, indicating the increased aggregation state of AuNPs (Fig. S1, ESI†).19,20 UV-Vis studies provided quantitative results, which showed that the absorbance at 520 nm gradually decreased while the absorbance at 650 nm increased with the increase of β-glucosidase concentration (Fig. S2, ESI†). This red shift in the SPR absorption indicated the formation of aggregates of AuNPs.21 The absorbance ratio (A650/A520) increased up to a steady value with the reaction time, and reached a plateau within 15 min. Therefore, the reaction time for this enzymatic reaction was set at 20 min.
For an inhibitor-screening assay of β-glucosidase, the procedure is similar to the activity assay. In a typical experiment, β-glucosidase (10 U L−1) was initially incubated with heavy metals or surfactants (1.0 mM) in a citrate–phosphate buffer (pH 5.0), and then the mixture was incubated at 30 °C for 20 min. The pretreated β-glucosidase solution (0.1 mL) was incubated with nanocomposites solution (2.0 mL) for another 20 min. Finally, the UV-Vis absorption spectra of the mixture solution were collected. The residual activity of β-glucosidase was calculated based on the measured A650/A520 ratio as mentioned above, and the data were expressed as a percentage of the enzyme activity without the heavy metal or surfactant.
3. Results and discussion
Fig. 1 showed the change in the absorbance ratio (A650/A520) of nanocomposites as a function of β-glucosidase concentration. On the basis of the spectral shift, β-glucosidase concentrations down to 3.0 U L−1 can be easily quantified with nanocomposites. The β-glucosidase activity is linear with the absorbance ratio (A650/A520) in the range of 3.0–100.0 U L−1 (100.48 to 102.0 U L−1). The corresponding regression equation is:| y = 0.5643x − 0.0586 |
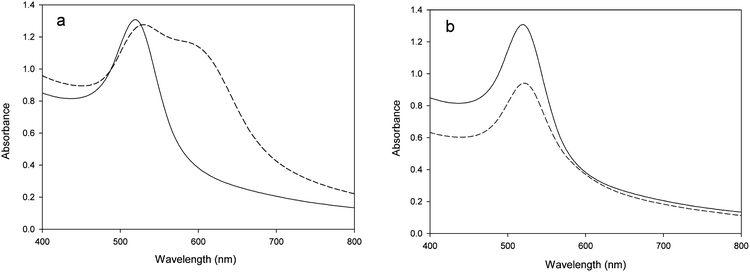
Moreover, to validate a potential application of the nanocomposites, we applied it to evaluate the inhibitory effect of heavy metals and surfactants on β-glucosidase. Inhibitors can influence the activity of β-glucosidase; thus, the aggregation of nanocomposites will become slow, and the absorption variation correspondingly changes. As shown in Fig. 2a, after the detection of enzyme without inhibitor, an increased absorbance at 650 nm and a decreased absorbance at 520 nm were observed in the UV-Vis spectrum of nanocomposites. In the presence of an efficient β-glucosidase inhibitor (e.g., Hg2+), a different spectral shift occurred after the addition of β-glucosidase into the nanocomposites solution. No obvious increased absorbance at 650 nm was observed due to inhibition of the enzymatic activity (Fig. 2b).
Transmission electron microscopic observation also indicated a difference in aggregation, which was induced by the β-glucosidase reaction in the presence and absence of an efficient inhibitor. As shown in Fig. 3b, the enzyme digestion induced the consumption of cellobiose and resulted in the aggregation of nanocomposites. In the presence of a sufficient amount of inhibitor (1.0 mM Hg2+), no significant aggregation of nanocomposites could be observed (Fig. 3c), which is similar to the results of the nanocomposites solution without enzyme treatment (Fig. 3a).
The effect of 1 mM concentration of CoCl2·6H2O, HgCl2, MnSO4·H2O, Pb(NO3)2, sodium dodecylsulphate (SDS) or cetyltrimethyl ammonium bromide (CTAB) on the activity of β-glucosidase is shown in Fig. 4. As can be seen, Co2+ (110%) and Mn2+ (103%) had a small stimulatory effect, and the β-glucosidase activity increased most in the presence of Pb2+. However, Hg2+ had a strong inhibitory effect, which reduced the β-glucosidase activity down to 37%. The anionic surfactant (SDS) revealed a stronger inhibitory effect than the cationic surfactant (CTAB). The results are consistent with the conclusions reported by previous studies, in which p-nitrophenyl-β-D-glucopyranoside (ρNPG) is used as the substrate of β-glucosidase.6–9 Moreover; the control experiment was conducted by mixing the nanocomposites solution with heavy metal or surfactant solution (1 mM). The color change and UV-Vis absorption spectrum of the nanocomposites were analyzed, and the results indicated that these chemicals did not influence the stability of the nanocomposites (data not shown). From these results, it is promising to apply the nanocomposites to the screening of β-glucosidase inhibitors.
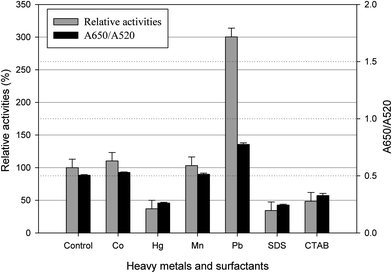 | ||
| Fig. 4 Effect of heavy metals and surfactants on the enzymatic activity of β-glucosidase (10 U L−1). Control represents the β-glucosidase without heavy metals or surfactants. | ||
4. Conclusions
In summary, the activity of β-glucosidase was successfully determined by using gold–cellobiose nanocomposites. In addition, the nanocomposites have potential for further application in the screening of β-glucosidase inhibitors. The authors believe that the proposed method has the potential to be developed as a platform for the screening of inhibitors of other polysaccharide-sensitive enzymes due to its simplicity, low cost and rapidity.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (51039001, 50808073, and 51278176), the Environmental Protection Technology Research Program of Hunan (2007185), the Young Teacher Growth Program of Hunan University and the Research Fund for the Doctoral Program of Higher Education of China (20100161110012), Hunan Provincial Natural Science Foundation of China (13JJ4107), the Hunan Provincial Innovation Foundation For Postgraduate (CX2012B137), Zhejiang Provincial Key Laboratory of Solid Waste Treatment and Recycling open fund (SWTR-2012-07), Shanghai Tongji Gao Tingyao Environmental Science & Technology Development Foundation (STGEF).References
- X. L. Shen and L. M. Xia, Process Biochem., 2004, 39, 1363–1367 CrossRef CAS.
- S. Ghorai, S. Chowdhury, S. Pal, S. P. Banik, S. Mukherjee and S. Khowala, Carbohydr. Res., 2010, 345, 1015–1022 CrossRef CAS PubMed.
- S. Ghorai, S. Mukherjee, S. Mukherjee and S. Khowala, Biotechnol. Bioprocess Eng., 2011, 16, 297–304 CrossRef CAS PubMed.
- L. P. V. Calsavara, F. F. D. Moraes and G. M. Zanin, Appl. Biochem. Biotechnol., 2011, 91-93, 615–626 CrossRef.
- S. Das, D. Berke-Schlessel, H. F. Ji, J. Mcdonough and Y. Wei, J. Mol. Catal. B: Enzym., 2011, 70, 49–54 CrossRef CAS PubMed.
- T. Y. Ghiou, Y. H. Lin, N. W. Su and M. H. Lee, J. Agric. Food Chem., 2010, 58, 8872–8878 CrossRef PubMed.
- L. Kredics, Z. Antal, L. Manczinger and E. Nagy, Lett. Appl. Microbiol., 2001, 33, 112–116 CrossRef CAS.
- M. C. Hsieh and T. L. Graham, Phytochemistry, 2011, 58, 995–1005 CrossRef.
- T. Kriksson, J. Börjesson and F. Tjerneld, Enzyme Microb. Technol., 2002, 31, 353–364 CrossRef.
- G. Hu, J. A. Heitmann Jr and O. J. Rojas, Anal. Chem., 2009, 81, 1872–1880 CrossRef CAS PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- M. Generoso, M. De Rosa, R. De Rosa, L. De magistris, M. Secondulfo, R. Fiandra, R. Carratù and M. Cartenì, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2003, 783, 349–357 CrossRef CAS.
- H. Z. Huang and X. R. Yang, Biomacromolecules, 2004, 5, 2340–2346 CrossRef CAS PubMed.
- K. Esumi, N. Takei and T. Yoshimura, Colloids Surf., B, 2003, 32, 117–123 CrossRef CAS.
- Z. M. Qi, H. S. Zhou, N. K. Matsuda, I. Honma, K. Shimada, A. Takatsu and K. Kato, J. Phys. Chem. B, 2004, 108, 7006–7011 CrossRef CAS.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS PubMed.
- J. H. He, T. Kunitake and A. Nakao, Chem. Mater., 2003, 15, 4401–4406 CrossRef CAS.
- J. H. He, T. Kunitake and T. Watanable, Chem. Commun., 2005, 795–796 RSC.
- Y. C. Chuang, J. C. Li, S. H. Chen, T. Y. Liu, C. H. Kuo, W. T. Huang and C. S. Lin, Biomaterials, 2010, 31, 6087–6095 CrossRef CAS PubMed.
- Z. G. Chen, Z. Wang, J. H. Chen, S. B. Wang and X. P. Huang, Analyst, 2012, 137, 3132–3137 RSC.
- L. H. Wang, X. F. Liu, X. F. Hu, S. P. Song and C. H. Fan, Chem. Commun., 2006, 3780–3782 RSC.
- D. Robinson, Biochem. J., 1956, 63, 39–44 CAS.
- P. W. Stege, G. A. Messina, G. Bianchi, R. A. Olsina and J. Raba, Anal. Bioanal. Chem., 2010, 397, 1347–1353 CrossRef CAS PubMed.
- D. L. Huang, G. M. Zeng, C. L. Feng, S. Hu, X. Y. Jiang, L. Tang, F. F. Su, Y. Zhang, W. Zeng and H. L. Liu, Environ. Sci. Technol., 2008, 42, 4946–4951 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ay41233d |
| This journal is © The Royal Society of Chemistry 2014 |