Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemiluminescence detection with water-soluble iridium(III) complexes containing a sulfonate-functionalised ancillary ligand†
Josephine
Truong
a,
Kara B.
Spilstead
a,
Gregory J.
Barbante
a,
Egan H.
Doeven
a,
David J. D.
Wilson
b,
Neil W.
Barnett
a,
Luke C.
Henderson
ac,
Jarrad M.
Altimari
a,
Samantha C.
Hockey
a,
Ming
Zhou
d and
Paul S.
Francis
*a
aCentre for Chemistry and Biotechnology, School of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, 75 Pigdons Road, Waurn Ponds, Victoria 3216, Australia. E-mail: paul.francis@deakin.edu.au
bDepartment of Chemistry, La Trobe Institute for Molecular Sciences, La Trobe University, Victoria 3086, Australia
cInstitute for Frontier Materials, Deakin University, 75 Pigdons Road, Waurn Ponds, Victoria 3216, Australia
dSunaTech Inc., BioBAY, Suzhou Industrial Park, Suzhou, Jiangsu 215123, P. R. China
First published on 12th September 2014
Abstract
The chemiluminescence from four cyclometalated iridium(III) complexes containing an ancillary bathophenanthroline-disulfonate ligand exhibited a wide range of emission colours (green to red), and in some cases intensities that are far greater than the commonly employed benchmark reagent, [Ru(bpy)3]2+. A similar complex incorporating a sulfonated triazolylpyridine-based ligand enabled the emission to be shifted into the blue region of the spectrum, but the responses with this complex were relatively poor. DFT calculations of electronic structure and emission spectra support the experimental findings.
Introduction
Cyclometalated iridium(III) complexes have been widely explored as electrochemiluminescence (ECL) reagents1–9 because of their wide range of emission colours and high luminescence efficiencies, which offer greater sensitivity than the benchmark tris(2,2′-bipyridine)ruthenium(II) ([Ru(bpy)3]2+) reagent, and the ability to create multi-coloured ECL systems.10–14 Preliminary studies suggest that cyclometalated iridium(III) complexes could also provide superior chemiluminescence detection of various analytes,15–21 but the limited solubility of many commercially available iridium(III) complexes in aqueous solution has restricted their application.We have previously demonstrated the use of the highly polar bathophenanthroline-disulfonate (BPS) as an ancillary ligand in a cyclometalated iridium(III) complex to increase its solubility in aqueous solution.15 Moreover, we showed that bis(2-phenylpyridine-C2,N)(bathophenanthroline-disulfonate)iridium(III) ([Ir(ppy)2(BPS)]−, Fig. 1a: 1) provided greater sensitivity (in terms of the calibration gradient) than [Ru(bpy)3]2+ for the determination of oxalate, but the ruthenium-based reagent still provided better limits of detection due to its lower blank responses.15 Investigations with the difluoro-phenylpyridine analogue, [Ir(df-ppy)2(BPS)]− (2), revealed significant differences in the selectivity of light producing reactions of 1 and 2.17 Moreover, complex 2 provided a superior limit of detection for the pharmaceutical furosemide than that obtained using [Ru(bpy)3]2+. Following the publication of this work,15,17 these and other bis-cyclometalated iridium complexes containing an ancillary BPS ligand (Fig. 1a; 1–4) have been utilised for a variety of luminescence-based applications5,12,22–24 and they are now commercially available.
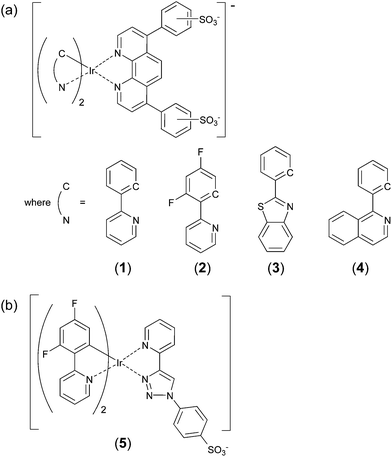 | ||
| Fig. 1 Iridium(III) complexes containing sulfonate-functionalised ligands. (a) Complexes containing the BPS ligand. The position of the sulfonate groups on BPS varies depending on the source of the ligand.22,25–27 In this study, the p–m′-regioisomer23 was used. (b) A complex containing the 1-phenylsulfonate-1,2,3-triazol-4-ylpyridine (STP) ligand. | ||
Herein we explore, for the first time, the chemiluminescence of recently commercialised complexes [Ir(bt)2(BPS)]− (3) and [Ir(piq)2(BPS)]− (4), in addition to a novel complex containing a sulfonated triazolylpyridine ancillary ligand (Fig. 1b; 5), in direct comparison with the previously studied [Ir(df-ppy)2(BPS)]− (2) and [Ru(bpy)3]2+ reagents.3,17
Results and discussion
We initially examined the chemiluminescence of complexes 2–4 and [Ru(bpy)3]2+ dissolved in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
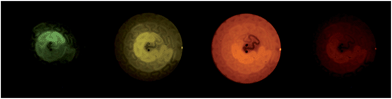
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 acetonitrile–water, upon reaction with cerium(IV) sulfate and three different analytes – ofloxacin, furosemide and codeine – using flow injection analysis, under the conditions described in our previous investigation.17 As shown in Table 1, these complexes exhibit a wide range of oxidation potentials and maximum emission wavelengths. The difference in overall emission colour (and reaction rates) could be clearly seen by visual examination of each chemiluminescence reaction with cerium(IV) and ofloxacin when the reactants were continuously merged in a dual-inlet serpentine28 flow-cell (Fig. 2).
50 acetonitrile–water, upon reaction with cerium(IV) sulfate and three different analytes – ofloxacin, furosemide and codeine – using flow injection analysis, under the conditions described in our previous investigation.17 As shown in Table 1, these complexes exhibit a wide range of oxidation potentials and maximum emission wavelengths. The difference in overall emission colour (and reaction rates) could be clearly seen by visual examination of each chemiluminescence reaction with cerium(IV) and ofloxacin when the reactants were continuously merged in a dual-inlet serpentine28 flow-cell (Fig. 2).
| Photoluminescence | Oxidation | ||
|---|---|---|---|
| λ em (nm) | ϕ PL 23 | E ox (V vs. Ag/AgCl)b | |
a 10 μM in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 acetonitrile–water at room temperature. Spectra were corrected for the wavelength dependence of the detector response and monochromator transmission.29
b 0.1 mM in acetonitrile–water containing 0.1 M TBAPF6 at room temperature, measured using a potentiostat with three-electrode configuration. 50 acetonitrile–water at room temperature. Spectra were corrected for the wavelength dependence of the detector response and monochromator transmission.29
b 0.1 mM in acetonitrile–water containing 0.1 M TBAPF6 at room temperature, measured using a potentiostat with three-electrode configuration.
|
|||
| [Ir(df-ppy)2(BPS)]− | 549 | 0.29 | 1.54 |
| [Ir(bt)2(BPS)]− | 573 | 0.40 | 1.36 |
| [Ru(bpy)3]2+ | 624 | 0.06 | 1.23 |
| [Ir(piq)2(BPS)]− | 632, 595 | 0.10 | 1.19 |
For quantitative comparison, the reagents (1 × 10−5 M) were injected into the analyte solution (1 × 10−6 M), which merged with the oxidant (1 × 10−3 M in 0.05 M H2SO4) in a T-piece just prior to entering a transparent PTFE coil flow-cell mounted in front of a photomultiplier tube. As observed in our previous work under these conditions,17 the green-light emitting [Ir(df-ppy)2(BPS)]− complex gave the greatest chemiluminescence signals (and signal-to-blank (S/B) ratios) with furosemide (Table 2). However, the current work revealed that [Ir(bt)2(BPS)]− provided greater chemiluminescence signals (and S/B ratios) with ofloxacin and codeine, in addition to the second greatest values with furosemide. This is considerably different to the reported order of ‘oxidative–reduction’ ECL intensities ([Ru(bipy)3]2+ (1) > [Ir(piq)2(BPS)]− (0.079) > [Ir(bt)2(BPS)]− (0.016) > [Ir(df-ppy)2(BPS)]− (0.001)) at relatively low metal-complex concentration with tri-n-propylamine co-reactant in buffered aqueous solution, despite the similarities in their light-producing reaction pathways.30,31 The complex exhibiting the highest photoluminescence quantum yield ([Ir(bt)2(BPS)]−, see Table 1) generally gave the largest chemiluminescence intensities, but overall, the correlation between photoluminescence quantum yields and chemiluminescence intensity was poor.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 acetonitrile–water) injecteda into 1 μM analyte, and then merged with 1 mM cerium(IV) in 0.05 M H2SO4
50 acetonitrile–water) injecteda into 1 μM analyte, and then merged with 1 mM cerium(IV) in 0.05 M H2SO4
| Ofloxacin | Furosemide | Codeine | |
|---|---|---|---|
| a n = 3; the relative standard deviation (RSD) of replicate injections was generally below 2.5%. | |||
| [Ir(df-ppy)2(BPS)]− | 3.7 (28) | 3.3 (25) | 0.13 (1.0) |
| [Ir(bt)2(BPS)]− | 29 (51) | 3.1 (4.6) | 2.4 (2.7) |
| [Ru(bpy)3]2+ | 4.6 (15) | 0.7 (2.5) | 0.45 (1.6) |
| [Ir(piq)2(BPS)]− | 6.6 (12) | 1.1 (1.2) | 0.97 (1.6) |
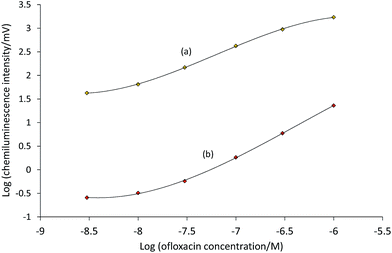
Ofloxacin calibrations prepared under these conditions exhibited a 7-fold steeper gradient, 2-fold higher intercept, and an order of magnitude superior limit of detection (3σ) of 3 × 10−9 M ofloxacin using [Ir(bt)2(BPS)]−, compared to that obtained using [Ru(bpy)3]2+.
When we increased the chemiluminescence reagent concentrations by orders of magnitude (to 1 mM, for example), the advantage of the iridium(III) complexes was diminished or even overcome. We have observed similar effects in the case of the [Ir(df-ppy)2(BPS)]− complex,17 and also for some related ruthenium(II) complexes,26 such as [Ru(BPS)3]4−. There is evidence to suggest that this (at least in part) arises from differences in the kinetics of the competing light-producing reactions of the oxidised reagent with the analyte and with the solvent.17 This effect explains the apparent discrepancy between the data in Table 2 and the relative intensities of light seen emanating from the flow-cells in Fig. 2, as the photographs were obtained using much higher concentrations of the reactants.
The presence of the BPS ligands in complexes 1–4 elicits a considerable bathochromic shift in the emission (compared to their homoleptic tris-cyclometalated analogues), towards a less sensitive region of the photodetector. To the naked eye, [Ir(ppy)3] emits green light (λmax = 530 nm),9 whereas the luminescence of [Ir(ppy)2(BPS)]− is orange (λmax = 628 nm).17 Similarly, the light from [Ir(df-ppy)3] is blue (λmax = 492 nm),9 but that from [Ir(df-ppy)2(BPS)]− is shifted into the green (λmax = 547 nm;17Fig. 2). To avoid this effect while maintaining reasonable solubility in water, we sought to prepare a sulfonated derivative of 1-phenyl-1,2,3-triazol-4-ylpyridine to use as an alternative ancillary ligand to BPS. In recent investigations of electrochemiluminescence detection with iridium(III) complexes,6,9 triazolylpyridine ligands have been shown to exert hypsochromic effects by stabilising the HOMO energy (compared to their homoleptic cyclometalated counterparts). In initial attempts to synthesise the sulfonated ligand and then form the iridium(III) complex, purification of the product from precursors was problematic. However, the target was readily obtained by first preparing the analogous thiol ligand (7) via an efficient two step synthetic sequence (Fig. 3a), then forming the iridium(III) complex (9; Fig. 3b), before oxidising the thiol with potassium peroxymonosulfate to form the desired sulfonate (5). The reagent was then directly prepared, without further isolation of the product, by appropriate dilution with an aqueous sulfuric acid solution.
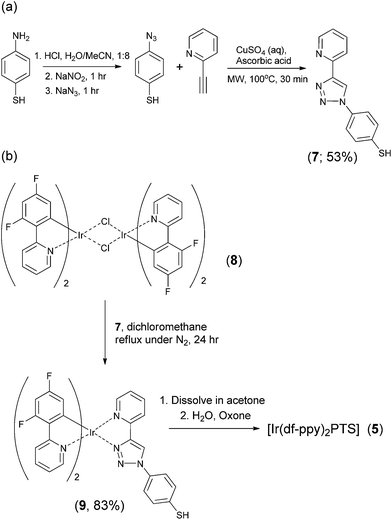 | ||
| Fig. 3 Synthesis of (a) the thiol ligand, and (b) the iridium(III) complex with an ancillary sulfonate-functionalised ligand. | ||
As expected, the emission maxima of [Ir(df-ppy)2(STP)] (λmax = 453 and 482 nm; Fig. 4a) occurred at much shorter wavelengths than those of [Ir(df-ppy)2(BPS)]− (λmax = 498 and 526 nm; Fig. 4b). For both complexes, the presence of vibronic fine structure is indicative of ligand-centred (LC) character.32
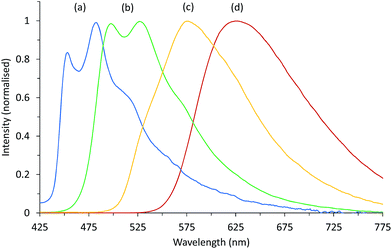 | ||
| Fig. 4 Photoluminescence emission spectrum of (a) [Ir(df-ppy)2(STP)], (b) [Ir(df-ppy)2(BPS)]−, (c) [Ir(bt)2(BPS)]−, and (d) [Ru(bpy)3]2+, at 10 μM in 0.05 M H2SO4. | ||
Comparison of the novel complex against [Ir(df-ppy)2(BPS)]−, [Ir(bt)2(BPS)]−, and [Ru(bpy)3]2+ in aqueous solution (without acetonitrile) was conducted using flow injection analysis, after optimisation of conditions (manifold configuration, flow rate, cerium(IV) concentration). The presence of only one sulfonate group on the ancillary ligand (and the fluorine groups on the phenylpyridine ligands) of [Ir(df-ppy)2(STP)] lowered its solubility in aqueous solution compared to the other complexes. We therefore limited the reagent concentration in these comparisons to 1 × 10−5 M. Greater signals and S/B ratios were obtained by injecting the reagent into a cerium(IV) stream which then merged with the analyte in the detector (which in this case was a GloCel™ with dual-inlet serpentine flow cell).28
As shown in Table 3, [Ir(df-ppy)2(STP)] generally gave the lowest chemiluminescence intensities, but its S/B ratios for furosemide were superior to those of other iridium complexes. Nevertheless, the emission from [Ir(df-ppy)2(STP)] is the shortest wavelength chemically-induced luminescence from a metal complex in aqueous solution reported to date. Similar to the above findings in mixed solvents, [Ir(bt)2(BPS)]− and [Ir(df-ppy)2(BPS)]− exhibited much higher chemiluminescence intensities than [Ru(bpy)3]2+ (Table 3). Furthermore, the considerable differences in their intensities were observed over a wide range of analyte concentrations (e.g.Fig. 5). Under these conditions, however, the conventional ruthenium(II) chelate gave superior S/B ratios, due to its much lower blank responses. Consequently, the limit of detection (3σ) of ofloxacin using [Ru(bpy)3]2+ (3 × 10−9 M) was better than that using [Ir(bt)2(BPS)]− (6 × 10−9 M).
| Ofloxacin | Furosemide | Codeine | |
|---|---|---|---|
| a n = 3; the RSD of replicate injections was generally below 2.5%. | |||
| [Ir(df-ppy)2(STP)] | 0.6 (1.3) | 3.2 (6) | 0.2 (0.5) |
| [Ir(df-ppy)2(BPS)]− | 91.4 (16) | 8.4 (1.4) | 17.9 (3) |
| [Ir(bt)2(BPS)]− | 536 (31) | 79.9 (4) | 16.6 (1.1) |
| [Ru(bpy)3]2+ | 24.9 (196) | 2.6 (20) | 0.8 (8) |
The differences in the blank signals of the reagents arise from: (i) the rate that each metal complex is oxidised by cerium(IV) (i.e. the proportion of the metal complex that is in its oxidised form within the detection zone); (ii) the relative rates of the chemiluminescent (blank) reaction of the oxidised metal-complexes with the acidic aqueous solvent;33 (iii) the efficiency that the corresponding excited state is generated;34 and (iv) the efficiency of the emission (i.e. photons emitted per excited luminophore molecules). The same or analogous considerations must be made when comparing the different responses of each reagent with the same analyte.
As the emissions corresponding to the solvent (blank) and analyte are both transient and may occur at different rates, the greatest S/B ratio observed in this flow-analytical system was dependent on the solution flow rate, which was optimised for each combination of analyte and reagent.
Theoretical calculations
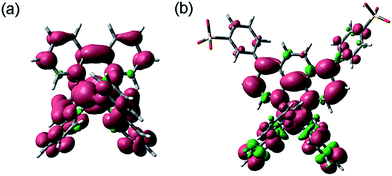
Density functional theory (DFT) calculations were employed to examine the electronic structure and nature of the radiative transition of each complex. The molecular orbitals (MOs) of [Ru(bpy)3]2+ are already well characterised,9,35,36 but we start our discussion with this complex for comparison purposes. As shown in Fig. 6a, the HOMO of [Ru(bpy)3]2+ is metal centred and the LUMO is distributed equally amongst the three bipyridine ligands. The triplet-state spin density (Fig. 7a) shares the same spatial extent as the singlet HOMO and LUMO, for which the lowest excited state may be described as metal-to-ligand charge-transfer (MLCT).35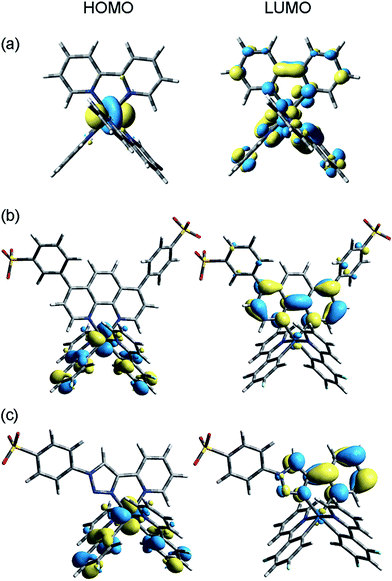 | ||
| Fig. 6 Ground-state singlet molecular energy surfaces of (a) [Ru(bpy)3]2+, (b) [Ir(df-ppy)2(BPS)]− and (c) [Ir(df-ppy)2(STP)]. | ||
Analysis of the MOs of [Ir(df-ppy)2(BPS)]− (Fig. 6b and 7b) is illustrative of the theoretical results for each of the [Ir(C^N)2(BPS)]− series (n.b.: frontier MO and triplet spin density surfaces for all complexes are included in Table S1 in ESI†). The singlet HOMO of [Ir(df-ppy)2(BPS)]− is principally composed of a mixture of the iridium d and the phenyl π orbitals, distributed equally across the two C^N-type df-ppy ligands. The LUMO is localised on the BPS ligand, predominantly on the phenanthroline moiety. The triplet spin density surface (Fig. 7b) shares the same spatial extent as the singlet LUMO and HOMO,37 which in this case leads to a description of the lowest energy excited state as having metal–ligand-to-ligand charge-transfer (MLLCT) character.
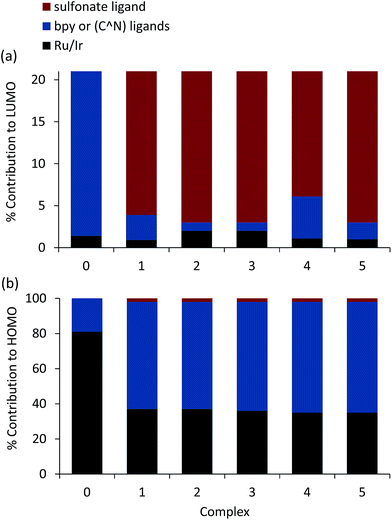
The results for the novel [Ir(df-ppy)2(STP)] complex (Fig. 6c) are analogous to those of the [Ir(C^N)2(BPS)]− series, where the singlet HOMO is composed of a mixture of the Ir d and the phenyl π orbitals of the df-ppy ligands, and the LUMO is localised on the sulfonate ligand. Mulliken population analysis of fragment contributions to the HOMO and LUMO (Fig. 8) highlighted the similarities of these iridium compounds. In each case, Ir contributes 35–37% of the HOMO while the phenyl (π) ring of the C^N ligand contributes ∼60%. The LUMO is almost exclusively composed of the sulfonate ligand (94–97%). The triplet spin density surface of [Ir(df-ppy)2(STP)] shares the same spatial extent as the singlet LUMO and HOMO.
For each of the iridium complexes under investigation, there is very little overlap between the singlet-state HOMO and LUMO (i.e. they are largely orthogonal), which indicates that the HOMO and LUMO energies can be independently ‘tuned’ by appropriate substitution of donor/acceptor groups on the C^N or sulfonate ligands, respectively. For example, the HOMO energies for [Ir(df-ppy)2(BPS)]− and [Ir(df-ppy)2(STP)]− are very similar (−6.07 and −6.11 eV), but the LUMO energies are −2.70 and −2.26 eV (Fig. S1; ESI†). That is, the common df-ppy ligand ensures little change to the HOMO properties, but modification of the sulfonate ligand significantly influences the LUMO.
The singlet–triplet transition energy of the complexes can be estimated from the difference between the triplet state highest singly occupied MO (HSOMO) and the singlet HOMO. For example, the B3LYP/def2-TZVP calculated energy difference for [Ir(bt)2(BPS)]− is 2.32 eV (534 nm). The experimental measurement is 576 nm, but this is only formally comparable to the theoretical results in the limit of low-temperature measurements (in this work all measurements were recorded at room temperature). Nevertheless, a general trend emerges that the rank order of calculated singlet–triplet energy gaps of the complexes matches that of the experimental results (Fig. S2; ESI†).
To probe the nature of the luminescence emission bands, TD-DFT calculations of the lowest-energy vertical emissions were carried out (estimated by singlet state TD-DFT calculations at the triplet-state optimised geometries; Table S2†). As discussed above, for the iridium compounds (Fig. 6 and 8), a HOMO–LUMO transition would be attributed to a mixture of MLCT and LLCT (HOMO is Ir d orbital and π orbital of the C^N ligand, LUMO is on the sulfonate ligand). However, the emission spectrum arises from a range of electronic transitions, and moreover, the HOMO–LUMO transition is not necessarily the dominant transition.
TD-DFT results indicate that the complexes that contain the df-ppy ligand exhibit emission bands with significant ligand-centred (LC) character. For [Ir(df-ppy)2(BPS)]−, the LC transition occurs within the BPS ligand (HOMO−1 to LUMO), while for [Ir(df-ppy)2(STP)]−, the LC transition occurs within the df-ppy ligand (HOMO to LUMO+1). Similarly, both the [Ir(bt)2(BPS)]− and [Ir(piq)2(BPS)]− complexes are predicted to exhibit some LC character. The [Ir(ppy)2(BPS)]− and [Ir(ppy)2(STP)]− complexes are not expected to show LC character, but are dominated by MLCT and LLCT transitions.
In general, emission bands from charge-transfer (CT) states are broad and featureless, while ligand-centred (LC) states typically give emissions with vibronic structure.32 The presence of vibronic structure in the emission spectra of [Ir(df-ppy)2(BPS)]− and [Ir(df-ppy)2(STP)]− (Fig. 4) is indicative of contributions from ligand-centred (LC) transitions,38,39 and is consistent with the theoretical results. The [Ir(bt)2(BPS)]− (Fig. 4) and [Ir(piq)2(BPS)]− (not shown) complexes also exhibit minor shoulder bands that are attributed to LC transitions. The emission bands of [Ir(ppy)2(BPS)]− and [Ir(ppy)2(STP)]− (not shown) are broad and featureless, consistent with MLCT and LLCT (MLLCT) character.
Mechanism considerations
The mechanism of chemiluminescent reactions of ruthenium(II) and related metal complexes with tertiary amines is well established (Fig. 9a).30,31,40 Oxidation of the tertiary amine by cerium(IV) or by the oxidised metal complex (M+) initially forms an aminium radical cation that decomposes to form a highly reductive alkyl radical species. The reaction of this intermediate with the oxidised metal complex can generate the electronically excited emissive species.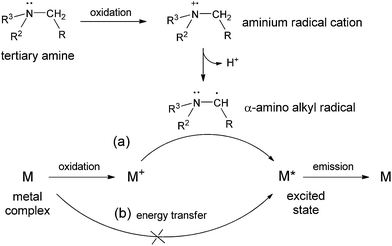 | ||
| Fig. 9 (a) Generalised mechanism of the chemiluminescence reactions of transition metal complexes with tertiary amines,30,31,40 and (b) an alternative light producing pathway involving energy transfer from an excited intermediate derived from the tertiary amine. | ||
We also observed a weak emission of light from the reaction of cerium(IV) with ofloxacin, in the absence of the metal complex, indicating that another electronically excited species (derived from the tertiary amine compound) can be generated in this reaction. In the presence of the metal complex, the contribution of the direct emission from the alternative excited state species to the overall chemiluminescence would not be significant. However, it is possible that the alternative excited species is capable of transferring energy to the more efficient metal complex luminophore (Fig. 9b), and this alternative light producing pathway could make a significant contribution to the overall emission.
To test the contribution of this alternative light-producing pathway, we replaced the metal complexes with two efficient luminophores, rhodamine B and quinine. These compounds have commonly been used to sensitise the weak chemiluminescence oxidation of various organic compounds with strong inorganic oxidants such as cerium(IV), bromate and permanganate.41–45 However, in this case, no significant emission from either luminophore was observed, indicating that the postulated energy transfer pathway (Fig. 9b) does not make a significant contribution to the chemiluminescence reaction of metal complexes.
The increased sensitivity of certain iridium complex reagents compared to [Ru(bpy)3]2+ was therefore largely attributed to a combination of their greater reactivity with the analytes after oxidation, in addition to superior excitation and/or luminescence efficiencies. Moreover, the observed analyte dependence of these effects highlights the relative importance of the reactivity of the respective oxidised complex towards the analyte to generate the radical intermediates, and towards the α-amino alkyl radical as the source of chemi-excitation.
Experimental
Instrumentation
Chemicals and reagents
Bis(2-phenylbenzothiazole)(bathophenanthrolinedisulfonate)-iridium(III); bis(2-(difluorophenyl)pyridine)(bathophenanthro-linedisulfonate)iridium(III); and bis(1-phenylisoquinoline)-(bathophenanthrolinedisulfonate)iridium(III) were provided by SunaTech (Suzhou, P. R. China). In each of these complexes, the BPS ligand was the para-, meta′-isomer.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PET spirits (1
PET spirits (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) and the product collected in a Hirsch funnel to give a light brown powder (268 mg, 53%). Analysis of the solid material by 1H NMR spectroscopy showed the desired compound in >95% purity. 1H NMR (d6-DMSO, 270 MHz): δ 9.34 (1H, s, Ar-H), 8.67–8.63 (1H, m, Ar-H), 8.14–8.06 (3H, m, Ar-H), 7.95 (1H, td, J = 8.1, 2.7 Hz, Ar-H), 7.81 (2H, d, J = 8.1 Hz, Ar-H), 7.43–7.37 (1H, m, Ar-H), SH not observed; 13C NMR (d6-DMSO, 67.5 MHz): δ 150.3, 149.9, 148.8, 137.9, 136.6, 136.4, 129.1, 123.9, 121.9, 121.8, 120.4.
10 v/v) and the product collected in a Hirsch funnel to give a light brown powder (268 mg, 53%). Analysis of the solid material by 1H NMR spectroscopy showed the desired compound in >95% purity. 1H NMR (d6-DMSO, 270 MHz): δ 9.34 (1H, s, Ar-H), 8.67–8.63 (1H, m, Ar-H), 8.14–8.06 (3H, m, Ar-H), 7.95 (1H, td, J = 8.1, 2.7 Hz, Ar-H), 7.81 (2H, d, J = 8.1 Hz, Ar-H), 7.43–7.37 (1H, m, Ar-H), SH not observed; 13C NMR (d6-DMSO, 67.5 MHz): δ 150.3, 149.9, 148.8, 137.9, 136.6, 136.4, 129.1, 123.9, 121.9, 121.8, 120.4.
Computational methods
DFT calculations were carried out within the Gaussian 09 suite of programs.47 Ground and triplet state geometries were optimised in the absence of solvent with the mPW1PW91 (ref. 47 and 48) functional in conjunction with the def2-SVP basis set and associated core potential.49 The mPW1PW91 functional has previously been demonstrated to yield reliable results for such systems.36,50 Stationary points were characterised as minima by calculating the Hessian matrix analytically at the same level of theory. All structures are minima with no imaginary frequencies. Due to difficulties with the D3 symmetry triplet state of [Ru(bpy)3]2+, a previously reported51 B3PW91/LANL2DZ calculated structure was used. Single-point energy calculations were carried out with the B3LYP functional52–54 and def2-TZVP basis set and core potential.49 TD-DFT calculations of emission bands were calculated at the B3LYP/def2-SVP level of theory as singlet states at the triplet-state optimised geometry, which represents a vertical triplet–singlet transition; 20 singlet and triplet states were calculated with TD-DFT. The polarisable continuum model (PCM)55 self-consistent reaction field (SCRF) was used to model solvent effects at the gas-phase optimised geometries with a solvent of acetonitrile or water, for consistency with the experimental system. The water and acetonitrile solvent results were almost identical; hence only water solvent results are presented. An SCF convergence criterion of 10−8 a.u. was employed throughout. Molecular orbital analysis was carried out with the AOMix program.56Conclusions
Chemiluminescence detection with iridium(III) complexes containing an ancillary BPS ligand provided a wide range of emission colours (green to red) from respective excited states. All complexes exhibited MLLCT emission bands, but [Ir(df-ppy)2(BPS)]− (and to a much lesser extent, [Ir(bt)2(BPS)]− and [Ir(piq)2(BPS)]−) also demonstrated LC emission character. Unlike previous comparisons of ECL, greater chemiluminescence responses were often obtained using the green-light emitting [Ir(df-ppy)2(BPS)]− and yellow-light emitting [Ir(bt)2(BPS)]− complexes than [Ru(bpy)3]2+. Most notably, the chemiluminescence response of each analyte with [Ir(bt)2(BPS)]− (and cerium(IV)) was over an order of magnitude greater than those of [Ru(bpy)3]2+ in acidic aqueous solution. However, the blank responses (from the competing reaction of the oxidised complex with the solvent) were also greater, limiting the translation of the enhanced sensitivity to superior limits of detection. An iridium(III) complex containing an ancillary STP ligand exhibited a blue emission, again attributable to MLLCT and LC transitions, but the chemiluminescence intensities of this complex were poor compared to [Ru(bpy)3]2+ and the other iridium complexes. Nevertheless, these findings have created new directions for the development of water-soluble iridium(III) complexes as chemiluminescence reagents.Acknowledgements
This research was funded by the Australian Research Council (FT100100646). We thank the Victorian Partnership for Advanced Computing (VPAC), the National Computational Infrastructure National Facility (NCI-NF) and La Trobe University for computing resources.Notes and references
- A. Kapturkiewicz and G. Angulo, Dalton Trans., 2003, 3907–3913 RSC.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- W. Miao, Chem. Rev., 2008, 108, 2506–2553 CrossRef CAS PubMed.
- S. Zanarini, E. Rampazzo, S. Bonacchi, R. Juris, M. Marcaccio, M. Montalti, F. Paolucci and L. Prodi, J. Am. Chem. Soc., 2009, 131, 14208–14209 CrossRef CAS PubMed.
- R. V. Kiran, C. F. Hogan, B. D. James and D. J. D. Wilson, Eur. J. Inorg. Chem., 2011, 4816–4825 CrossRef CAS.
- S. Zanarini, M. Felici, G. Valenti, M. Marcaccio, L. Prodi, S. Bonacchi, P. Contreras-Carballada, R. M. Williams, M. C. Feiters, R. J. M. Nolte, L. De Cola and F. Paolucci, Chem.–Eur. J., 2011, 17, 4640–4647 CrossRef CAS PubMed.
- K. N. Swanick, S. Ladouceur, E. Zysman-Colman and Z. Ding, Chem. Commun., 2012, 48, 3179–3181 RSC.
- K. N. Swanick, S. Ladouceur, E. Zysman-Colman and Z. Ding, Angew. Chem., Int. Ed., 2012, 51, 11079–11082 CrossRef CAS PubMed.
- G. J. Barbante, E. H. Doeven, E. Kerr, T. U. Connell, P. S. Donnelly, J. M. White, T. Lópes, S. Laird, C. F. Hogan, D. J. D. Wilson, P. J. Barnard and P. S. Francis, Chem.–Eur. J., 2014, 20, 3322–3332 CrossRef CAS PubMed.
- D. Bruce and M. M. Richter, Anal. Chem., 2002, 74, 1340–1342 CrossRef CAS.
- B. D. Muegge and M. M. Richter, Anal. Chem., 2004, 76, 73–77 CrossRef CAS.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, C. F. Hogan, N. W. Barnett and P. S. Francis, Angew. Chem., Int. Ed., 2012, 51, 4354–4357 CrossRef CAS PubMed.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, P. S. Francis, N. W. Barnett and C. F. Hogan, Chem. Sci., 2013, 4, 977–982 RSC.
- E. H. Doeven, G. J. Barbante, E. Kerr, C. F. Hogan, J. A. Endler and P. S. Francis, Anal. Chem., 2014, 86, 2727–2732 CrossRef CAS PubMed.
- R. V. Kiran, E. M. Zammit, C. F. Hogan, B. D. James, N. W. Barnett and P. S. Francis, Analyst, 2009, 134, 1297–1298 RSC.
- J. M. Terry, E. M. Zammit, T. Slezak, N. W. Barnett, D. C. Olson, D. K. Wolcott, D. L. Edwards and P. S. Francis, Analyst, 2011, 136, 913–919 RSC.
- E. M. Zammit, N. W. Barnett, L. C. Henderson, G. A. Dyson, M. Zhou and P. S. Francis, Analyst, 2011, 136, 3069–3072 RSC.
- F. Wu, B. Tong and Q. Zhang, Anal. Sci., 2011, 27, 529–533 CrossRef CAS.
- F. Wu, B. Tong, X. Wei and L. Chen, Luminescence, 2012, 27, 519–523 CrossRef CAS PubMed.
- Y. P. Dong, L. Huang, B. H. Tong, M. J. Shi, W. B. Zhang and Q. F. Zhang, Luminescence, 2012, 27, 262–267 CrossRef CAS PubMed.
- Y. P. Dong, M. J. Shi, B. H. Tong and Q. F. Zhang, Luminescence, 2012, 27, 414–418 CrossRef CAS PubMed.
- C. Liu, L. Yu, Y. Liu, L. Fang and M. Zhou, Magn. Reson. Chem., 2011, 49, 816–823 CrossRef CAS PubMed.
- L. Yu, Z. Huang, Y. Liu and M. Zhou, J. Organomet. Chem., 2012, 718, 14–21 CrossRef CAS PubMed.
- J. Jia, H. Fei and M. Zhou, Electrophoresis, 2012, 33, 1397–1401 CrossRef CAS PubMed.
- S. A. De Pascali, D. Migoni, P. Papadia, A. Muscella, S. Marsigliante, A. Ciccarese and F. P. Fanizzi, Dalton Trans., 2006, 5077–5087 RSC.
- G. P. McDermott, E. M. Zammit, E. K. Bowen, M. M. Cooke, J. L. Adcock, X. A. Conlan, F. M. Pfeffer, N. W. Barnett, G. A. Dyson and P. S. Francis, Anal. Chim. Acta, 2009, 634, 222–227 CrossRef CAS PubMed.
- L. Della Ciana, S. Zanarini, R. Perciaccante, E. Marzocchi and G. Valenti, J. Phys. Chem. C, 2010, 114, 3653–3658 CAS.
- J. M. Terry, J. L. Adcock, D. C. Olson, D. K. Wolcott, C. Schwanger, L. A. Hill, N. W. Barnett and P. S. Francis, Anal. Chem., 2008, 80, 9817–9821 CrossRef CAS PubMed.
- P. S. Francis, J. L. Adcock and N. W. Barnett, Spectrochim. Acta, Part A, 2006, 65, 708–710 CrossRef PubMed.
- W. Miao, J.-P. Choi and A. J. Bard, J. Am. Chem. Soc., 2002, 124, 14478–14485 CrossRef CAS PubMed.
- C. M. Hindson, G. R. Hanson, P. S. Francis, J. L. Adcock and N. W. Barnett, Chem.–Eur. J., 2011, 17, 8018–8022 CrossRef CAS PubMed.
- F. Neve, M. La Deda, A. Crispini, A. Bellusci, F. Puntoriero and S. Campagna, Organometallics, 2004, 23, 5856–5863 CrossRef CAS.
- R. D. Gerardi, N. W. Barnett and P. Jones, Anal. Chim. Acta, 1999, 388, 1–10 CrossRef CAS.
- P. S. Francis and J. L. Adcock, Chromatogr. Sci. Ser., 2012, 104, 221–250 CAS.
- S. Campagna, F. Puntoriero, F. Nastasi, G. Bergamini and V. Balzani, Top. Curr. Chem., 2007, 280, 117–214 CrossRef CAS.
- G. J. Barbante, C. F. Hogan, D. J. D. Wilson, N. A. Lewcenko, F. M. Pfeffer, N. W. Barnett and P. S. Francis, Analyst, 2011, 136, 1329–1338 RSC.
- A. B. Tamayo, S. Garon, T. Sajoto, P. I. Djurovich, I. M. Tsyba, R. Bau and M. E. Thompson, Inorg. Chem., 2005, 44, 8723–8732 CrossRef CAS PubMed.
- Q. Zhao, M. Yu, L. Shi, S. Liu, C. Li, M. Shi, Z. Zhou, C. Huang and F. Li, Organometallics, 2010, 29, 1085–1091 CrossRef CAS.
- E. Orselli, R. Q. Albuquerque, P. M. Fransen, R. Fröhlich, H. M. Janssen and L. De Cola, J. Mater. Chem., 2008, 18, 4579–4590 RSC.
- Y. Yuan, S. Han, L. Hu, S. Parveen and G. Xu, Electrochim. Acta, 2012, 82, 484–492 CrossRef CAS PubMed.
- J. S. Lancaster, P. J. Worsfold and A. Lynes, Analyst, 1989, 144, 1659–1661 RSC.
- F. A. Aly, N. A. Alarfaj and A. A. Alwarthan, Anal. Chim. Acta, 1998, 358, 255–262 CrossRef CAS.
- B. Li, Z. Zhang, M. Liu and C. Xu, Anal. Bioanal. Chem., 2003, 377, 1212–1216 CrossRef CAS PubMed.
- P. S. Francis, A. J. Brown, S. A. Bellomarino, A. M. Taylor, T. Slezak and N. W. Barnett, Luminescence, 2009, 24, 90–95 CrossRef CAS PubMed.
- J. L. Adcock, P. S. Francis and N. W. Barnett, J. Fluoresc., 2009, 19, 867–874 CrossRef CAS PubMed.
- M. Mydlak, C. Bizzarri, D. Hartmann, W. Sarfert, G. Schmid and L. De Cola, Adv. Funct. Mater., 2010, 20, 1812–1820 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- J. P. Perdew, in Electronic Structure of Solids '91, ed. P. Ziesche and H. Eschrig, Akademie Verlag, Berlin, 1991, pp. 11–20 Search PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- J. Lin, K. Wu and M. Zhang, J. Comput. Chem., 2009, 30, 2056–2063 CrossRef CAS PubMed.
- K. Nozaki, K. Takamori, Y. Nakatsugawa and T. Ohno, Inorg. Chem., 2006, 45, 6161–6178 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785–789 CrossRef CAS.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- S. I. Gorelsky, AOMix: Program for Molecular Orbital Analysis, Version 6.46, University of Ottawa, 2010, http://www.sg-chem.net/ Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional ground state singlet HOMO and LUMO and excited state triplet spin density surfaces; HOMO, LUMO and singlet–triplet transition energies; and Cartesian coordinates of optimized geometries. See DOI: 10.1039/c4an01366b |
| This journal is © The Royal Society of Chemistry 2014 |