Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Haptens, bioconjugates, and antibodies for penthiopyrad immunosensing†
Eric
Ceballos-Alcantarilla
b,
Antonio
Abad-Fuentes
a,
Vincenzo
Aloisio
b,
Consuelo
Agulló
b,
Antonio
Abad-Somovilla
b and
Josep V.
Mercader
*a
aDepartment of Biotechnology, Institute of Agrochemistry and Food Technology, Consejo Superior de Investigaciones Científicas (IATA-CSIC), Agustí Escardino 7, 46980 Paterna, València, Spain. E-mail: jvmercader@iata.csic.es
bDepartment of Organic Chemistry, Universitat de València, Doctor Moliner 50, 46100 Burjassot, València, Spain
First published on 26th August 2014
Abstract
Haptens, bioconjugates, and antibodies for highly sensitive immunochemical analysis of the new-generation fungicide penthiopyrad are described. Two haptens with equivalent carboxylated linkers were prepared, and the purified active esters were efficiently coupled to proteins. The results revealed slightly different antibody-eliciting capacities for the two synthetic derivatives. All of the produced antibodies were specific for penthiopyrad, and showed affinity values in the nanomolar range.
Penthiopyrad (Fig. 1) is a new-generation pyrazole carboxamide fungicide that interferes with fungal cell respiration in complex II of the cytochrome system by inhibiting the succinate dehydrogenase activity (Group 7).1 Penthiopyrad is active against many diseases caused by ascomycetes, deuteromycetes, and basidiomycetes,2 and due to its unique mode and site of action, it has shown great potential as complementary active principle in order to fight against fungi resistance to other frequently used fungicides such as strobilurins and sterol biosynthesis inhibitors.3,4 Penthiopyrad was first commercially registered in the United States in 2012 and it has been recently approved by the European Commission.5
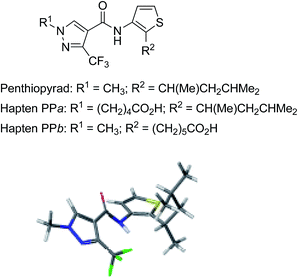 | ||
| Fig. 1 Chemical structures of penthiopyrad and the two synthetic haptens. Global minimum energy conformer of penthiopyrad calculated by CAChe/CONFLEX/MM3. | ||
Nowadays, antibody-based technologies constitute valuable complementary and/or alternative methods for chemical residue and contaminant analysis.6 In order to achieve good acceptance and widespread implementation of immunosensors in quality control laboratories, appropriate reagents are required and alternative analytical approaches are in demand. Therefore, research projects are needed aiming to increase the scientific knowledge about the processes and factors influencing the analytical properties of antibodies and the development of sensitive procedures.
For small chemicals, such as penthiopyrad, functionalized derivatives of the target molecule containing a linker arm and an activatable chemical group for conjugation to the carrier are usually essential.7–9 The main determinant groups of the target compound must be displayed unmodified in the immunizing conjugate. This rule can generally be achieved by introducing a carbohydrate aliphatic linear spacer at the appropriate site of the analyte.10 Moreover, the optimum hapten design will benefit not only antibody selectivity and affinity but also assay performance.11 The aim of this study was to investigate different derivatives of penthiopyrad with alternative linker tethering sites and to generate protein conjugates as well as selective and high-affinity antibodies in order to develop sensitive immunoassays for the rapid analysis of this fungicide.
Following those principles, two penthiopyrad haptens – hapten PPa and hapten PPb (Fig. 1) – were designed mimicking as much as possible the structural and electronic properties of the parent analyte. In each of them, the spacer arm was located at opposite positions of the penthiopyrad framework in order to favour alternative display orientations to the immune system. Hapten PPa came from the formal replacement of the pyrazole N-methyl group by a C5 carboxylated aliphatic linear chain, which presumably only implied a minimum modification of the electronic and steric properties of the parent compound, whereas in hapten PPb a similar linear spacer arm replaced the branched 4-methylpentan-2-yl substituent at the 2-position of the thiophene ring. In the latter case, there was a greater alteration of the penthiopyrad framework, especially from the steric point of view, but it only affected the proximal part of the hapten in the immunogenic conjugate.
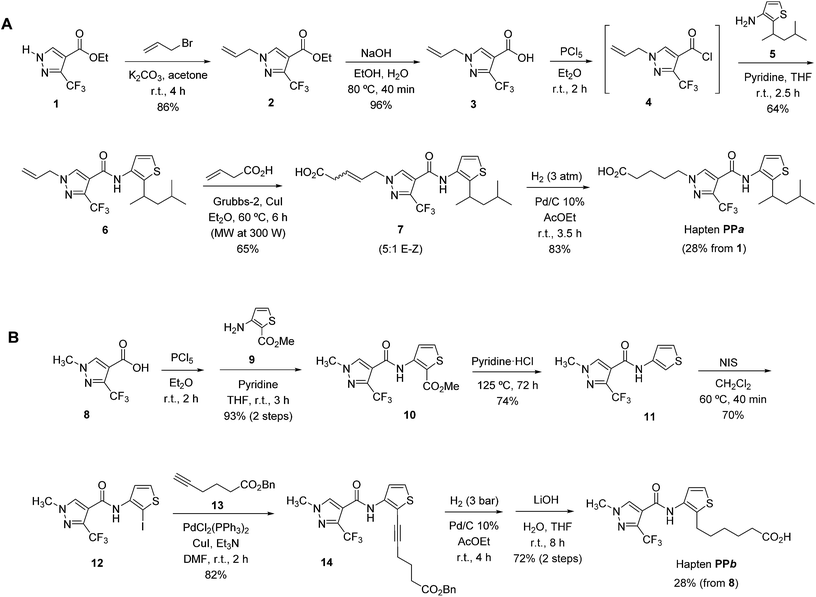
Since none of the two designed haptens can be prepared by chemical transformations from penthiopyrad or other structurally related accessible compounds, it was necessary to develop a total synthesis of both molecules from commercially available materials. The synthetic sequences developed for the preparation of haptens PPa and PPb are shown in Schemes 1A and B, respectively. Every intermediate of the synthesis as well as the final haptens were purified and fully characterized by spectroscopic methods (for details see the ESI, Schemes S1–S3†). A brief discussion of each of the synthetic procedures is also given in the ESI.† The synthesis of hapten PPa was accomplished via a six step sequence in 28% overall yield, while the synthesis of hapten PPb required seven reaction steps with a calculated overall yield of ca. 30%. In both cases, the overall yields can be considered very satisfactory due to the number and type of reactions that were involved, showing the effectiveness of the developed synthetic sequences.
Carboxyl-functionalized haptens were activated using N,N′-disuccinimidyl carbonate in acetonitrile following an adapted strategy that was optimized in previous studies for other compounds,12 which allowed a straightforward purification and characterization of the resulting active N-hydroxysuccinimidyl esters. Immunizing conjugates with BSA and assay conjugates with OVA and HRP were readily prepared by adding the corresponding pure active ester of the hapten over buffered protein solutions. Data for hapten activation and conjugation are given in the ESI.† Finally, covalent hapten-to-protein complexes were purified by size exclusion chromatography. This step-by-step approach avoided the formation of undesired secondary products during protein coupling, and therefore the same strategy could be employed both for immunizing and assay conjugate preparation, even for polyclonal antibody-based immunoassays. Moreover, since the conjugation reaction was more efficient, lower amounts of hapten were required. The number of conjugated hapten molecules was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/MS). The hapten-to-protein molar ratios (MR) obtained for the immunogens were 19.2 and 15.1 for conjugates BSA–PPa and BSA–PPb, respectively (Fig. S1 in the ESI†). The MRs for assay conjugates were 2.7 and 3.2 for conjugates OVA–PPa and OVA–PPb, respectively, and 2.9 and 2.2 for conjugates HRP–PPa and HRP–PPb, respectively (see Fig. S2 and S3 in the ESI†).
Animal manipulation was performed in compliance with the laws and guidelines of the Spanish Ministry of Agriculture, Food, and Environment. Antibodies were generated in rabbits by immunization with 0.3 mg of BSA–hapten conjugate using Freund's adjuvants. Partial antibody purification was performed by salting out twice. Further details are provided in the ESI.† Antibody-coated direct cELISAs and conjugate-coated indirect cELISAs were performed using homologous and heterologous conjugates as described in the ESI.† Mean absorbance values were plotted versus the logarithm of the analyte concentration, and assay sensitivity to penthiopyrad was estimated as the analyte concentration reducing 50% (IC50) the maximum absorbance (Amax).
From each penthiopyrad hapten, two polyclonal antibodies were obtained; namely, antibodies PPa#1 and PPa#2 from hapten PPa, and antibodies PPb#1 and PPb#2 from hapten PPb. Those antibodies were evaluated by checkerboard cELISA with the two hapten conjugates. Eight-point standard curves were prepared by a 10-fold serial dilution, including a blank point. A 10 mM penthiopyrad stock solution in N,N-dimethylformamide was used to prepare the first point of the standard curve. One penthiopyrad standard curve was run in every microplate column, and different antibody or conjugate concentrations were assayed in the same plate. For the direct format, three different antibody dilutions (1/3000, 1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 1/30
000, and 1/30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and four enzyme tracer concentrations (3, 10, 30, and 100 ng mL−1) were assayed in the same plate. For indirect competitive assays, plates were coated with the 100 or 1000 ng mL−1 OVA conjugate, and diverse antibody dilutions were assayed from 1/3000 to 1/300
000) and four enzyme tracer concentrations (3, 10, 30, and 100 ng mL−1) were assayed in the same plate. For indirect competitive assays, plates were coated with the 100 or 1000 ng mL−1 OVA conjugate, and diverse antibody dilutions were assayed from 1/3000 to 1/300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in one plate. Thus, a series of twelve inhibition curves was retrieved from every plate; each curve corresponding to a particular antibody and assay conjugate combination at a specific concentration.
000 in one plate. Thus, a series of twelve inhibition curves was retrieved from every plate; each curve corresponding to a particular antibody and assay conjugate combination at a specific concentration.
A sufficient assay signal (over 0.8) at a zero dose of analyte was achieved with every antibody/conjugate combination in both assay formats, with the only exception of antibody PPa#1 which did not bind the heterologous enzyme tracer HRP–PPb (Table 1). All assays showed negligible background signals, in spite of using the same coupling procedure for immunizing and assay conjugate preparation. The observed IC50 values were mostly in the low nanomolar range demonstrating the suitability of the prepared haptens and immunogens. The affinities for penthiopyrad of antibodies that were generated from hapten PPa were somewhat higher than those of the antibodies obtained from hapten PPb, indicating that hapten PPa was a better mimic of the target analyte as discussed previously for hapten design.
| Direct cELISA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ab | Tracer conjugate | |||||||
| HRP–PPa | HRP–PPb | |||||||
| [Ab]a | [T]b | A max | IC50 (nM) | [Ab] | [T] | A max | IC50 (nM) | |
| a Antibody dilution (×103). b Tracer concentration in ng mL−1. c Signal was lower than 0.8. d Conjugate concentration in ng mL−1. | ||||||||
| PPa#1 | 10 | 10 | 1.22 ± 0.16 | 4.39 ± 0.21 | 3 | 300 | nsc | — |
| PPa#2 | 10 | 10 | 1.24 ± 0.25 | 4.73 ± 0.62 | 3 | 300 | 0.99 ± 0.14 | 1.22 ± 0.16 |
| PPb#1 | 3 | 100 | 0.83 ± 0.14 | 13.79 ± 4.71 | 3 | 100 | 1.09 ± 0.23 | 7.05 ± 3.07 |
| PPb#2 | 3 | 100 | 0.80 ± 0.14 | 6.47 ± 1.08 | 10 | 30 | 0.88 ± 0.24 | 3.14 ± 0.99 |
| Indirect cELISA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ab | Coating conjugate | |||||||
| OVA–PPa | OVA–PPb | |||||||
| [Ab] | [C]d | A max | IC50 (nM) | [Ab] | [C] | A max | IC50 (nM) | |
| PPa#1 | 100 | 100 | 0.94 ± 0.12 | 3.25 ± 0.90 | 30 | 100 | 1.24 ± 0.09 | 3.29 ± 0.96 |
| PPa#2 | 100 | 100 | 0.95 ± 0.13 | 2.23 ± 0.36 | 30 | 100 | 1.47 ± 0.45 | 1.83 ± 0.14 |
| PPb#1 | 3 | 100 | 1.30 ± 0.03 | 6.06 ± 1.06 | 30 | 100 | 0.90 ± 0.14 | 22.37 ± 1.67 |
| PPb#2 | 10 | 100 | 1.27 ± 0.10 | 9.27 ± 3.97 | 30 | 100 | 1.88 ± 0.32 | 34.84 ± 4.10 |
The IC50 values of PPa-type antibodies could be lowered by using the heterologous conjugates with hapten PPb. Such improvement was also observed with PPb-type antibodies in the indirect assay format, but not in the direct format (Table 1). These results show the importance of preparing different functionalized haptens, not only for high affinity antibody production but also for sensitive assay development. Linker site heterologies are adequate for assay sensitivity improvement; however, for particular antibodies and assay formats, this could be a too drastic modification making it difficult to achieve sufficient signal. In both cELISAs, the best results were obtained with antibody PPa#2 combined with the heterologous conjugate; IC50 values of 1–2 nM with OVA–PPb or HRP–PPb, according to the assay format. The standard curves of the most sensitive immunoreagent combination by direct and indirect cELISA can be seen in Fig. S4 of the ESI.†
Antibody selectivity was studied by direct cELISA. Cross-reactivity (%) with other fungicides was determined from the quotient between the IC50 values for penthiopyrad and for the corresponding assayed compound, both in molar concentration units. As competitors, different pyrazole bioactive principles (pyraclostrobin, fluxapyroxad, fluopyram, and fluopicolide) and other common fungicides (azoxystrobin, boscalid, pyrimethanil, cyprodinil, fenhexamid, fludioxonil, and tebuconazole) were evaluated. For PPa-type antibodies, no inhibition was found at a concentration of 10 μM with any of the studied compounds. Regarding the two PPb-type antibodies, a moderate inhibition was observed only with fluxapyroxad, which contains a similar pyrazole carboxamide group to that of penthiopyrad – the calculated cross-reactivity was below 0.5% for both PPb-type antibodies. This result is in accordance with Landsteiner's principle,13 stating that antibody specificity is predominantly directed towards the hapten moieties positioned opposite to the linker tethering site. In fact, the spacer arm location in hapten PPb was distal to the pyrazole ring.
In conclusion, two haptens were designed with equivalent carboxylated linkers at opposite tethering sites of the target molecule. Haptens PPa and PPb were achieved in six and seven steps, respectively, by the total synthesis of the functionalized derivative. Purified active esters of the haptens were employed for efficiently preparing both the immunizing and the assay conjugates. High-affinity polyclonal antibodies to penthiopyrad were generated from both haptens. To the best of our knowledge, these are the first reported haptens, bioconjugates, and antibodies to this modern fungicide. Antibodies from hapten PPa displayed a moderately higher affinity and selectivity than those obtained from hapten PPb. The replacement of the branched aliphatic substituent at the thiophene ring (Fig. 1) by the linear hydrocarbon spacer arm (hapten PPb) did not hamper the generation of antibodies with IC50 values in the nanomolar range – probably because such minor alteration was located at a proximal position – though it was shown to be a slight hindrance to antibody affinity. Finally, antibody PPa#2 was identified as a promising candidate for sensitive immunosensor development and sample analysis.
Acknowledgements
This work was supported by the Spanish Ministerio de Economía y Competitividad (AGL2012-39965-C02-01/02) and cofinanced by FEDER funds. J.V.M. is hired by the Consejo Superior de Investigaciones Científicas (CSIC) under a Ramón y Cajal contract, cofinanced by Ministerio de Ciencia e Innovación and by the European Social Fund. E.C.-A. is a recipient of a predoctoral fellowship from the “Atracció de Talent, VLC-CAMPUS” program of the University of Valencia. The proteomic analysis was carried out in the SCSIE University of Valencia Proteomics Unit, a member of the ISCIII ProteoRedProteomics Platform. Open Access was financed by the Unit of Information Resources for Research (CSIC).Notes and references
- FRAC Code List 2013, http://www.frac.info, accessed April 2014.
- K. Culbreath, T. B. Brenneman, R. C. Kemerait Jr and G. G. Hammes, Pest Manage. Sci., 2009, 65, 66 CrossRef PubMed.
- K. L. Fairchild, T. D. Miles and P. S. Wharton, Crop Prot., 2013, 49, 31 CrossRef CAS PubMed.
- H. F. Avenot and T. J. Michailides, Crop Prot., 2010, 29, 643 CrossRef CAS PubMed.
- Commission Regulation (EU) No 1187/2013, Off. J. Eur. Union, 2013, L313/42, 1.
- J. M. Van Emon, J. AOAC Int., 2010, 93, 1681 CAS.
- M. Sathe, M. Dervini, G. Broadbent, A. Bodlenner, K. Charlton, B. Ravi, M. Rohmer, M. R. Sims and D. C. Cullen, Anal. Chim. Acta, 2011, 708, 97 CrossRef CAS PubMed.
- X. Cai, K. Tsuchikama and K. D. Janda, J. Am. Chem. Soc., 2013, 135, 2971 CrossRef CAS PubMed.
- Z. L. Xu, Y. D. Shen, T. M. Sun, K. Campbell, T. X. Tian, S. W. Zhang, H. T. Lei and T. M. Jiang, Talanta, 2013, 103, 306 CrossRef CAS PubMed.
- J. Parra, J. V. Mercader, C. Agulló, A. Abad-Fuentes and A. Abad-Somovilla, Tetrahedron, 2011, 67, 624 CrossRef CAS PubMed.
- J. Parra, J. V. Mercader, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, Anal. Chim. Acta, 2012, 715, 105 CrossRef CAS PubMed.
- F. A. Esteve-Turrillas, J. Parra, A. Abad-Fuentes, C. Agulló, A. Abad-Somovilla and J. V. Mercader, Anal. Chim. Acta, 2010, 682, 93 CrossRef CAS PubMed.
- K. Landsteiner, in The Specificity of Serological Reactions, Dover Publications, New York, revised edn, 1962 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General methods and instruments, experimental details of hapten synthesis (Schemes S1–S3), spectrometric characterization data, hapten activation and conjugation details, MALDI spectra of conjugates, antibody generation, competitive ELISA procedures, standard curves (Fig. S1), and 1H NMR spectra of haptens PPa and PPb. See DOI: 10.1039/c4an00828f |
| This journal is © The Royal Society of Chemistry 2014 |