Microchip electrophoresis with amperometric detection method for profiling cellular nitrosative stress markers
Abstract
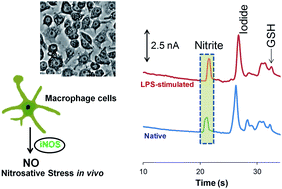
The overproduction of nitric oxide (NO) in cells results in nitrosative stress due to the generation of highly reactive species such as peroxynitrite and N2O3. These species disrupt the cellular redox processes through the oxidation, nitration, and nitrosylation of important biomolecules. Microchip electrophoresis (ME) is a fast separation method that can be used to profile cellular nitrosative stress through the separation of NO and nitrite from other redox-active intracellular components such as cellular antioxidants. This paper describes a ME method with electrochemical detection (ME-EC) for the separation of intracellular nitrosative stress markers in macrophage cells. The separation of nitrite, azide (interference), iodide (internal standard), tyrosine, glutathione, and hydrogen peroxide (neutral marker) was achieved in under 40 s using a run buffer consisting of 7.5 to 10 mM NaCl, 10 mM boric acid, and 2 mM TTAC at pH 10.3 to 10.7. Initially, NO production was monitored by the detection of nitrite (NO2−) in cell lysates. There was a 2.5- to 4-fold increase in NO2− production in lipopolysaccharide (LPS)-stimulated cells. The concentration of NO2− inside a single unstimulated macrophage cell was estimated to be 1.41 mM using the method of standard additions. ME-EC was then used for the direct detection of NO and glutathione in stimulated and native macrophage cell lysates. NO was identified in these studies based on its migration time and rapid degradation kinetics. The intracellular levels of glutathione in native and stimulated macrophages were also compared, and no significant difference was observed between the two conditions.
- This article is part of the themed collection: Probe and chip approaches to cell analysis

 Please wait while we load your content...
Please wait while we load your content...