 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced Raman multigas sensing – a novel tool for control and analysis of 13CO2 labeling experiments in environmental research
Robert
Keiner
ab,
Torsten
Frosch
*ab,
Tara
Massad
c,
Susan
Trumbore
c and
Jürgen
Popp
abd
aInstitute of Photonic Technology, Jena, Germany. E-mail: torsten.frosch@uni-jena.de
bFriedrich-Schiller University, Institute for Physical Chemistry, Jena, Germany
cMax-Plack-Institute for Biogeochemistry, Jena, Germany
dFriedrich-Schiller University, Abbe School of Photonics, Jena, Germany
First published on 30th April 2014
Abstract
Cavity-enhanced Raman multigas spectrometry is introduced as a versatile technique for monitoring of 13CO2 isotope labeling experiments, while simultaneously quantifying the fluxes of O2 and other relevant gases across a wide range of concentrations. The multigas analysis was performed in a closed cycle; no gas was consumed, and the gas composition was not altered by the measurement. Isotope labeling of plant metabolites via photosynthetic uptake of 13CO2 enables the investigation of resource flows in plants and is now an important tool in ecophysiological studies. In this experiment the 13C labeling of monoclonal cuttings of Populus trichocarpa was undertaken. The high time resolution of the online multigas analysis allowed precise control of the pulse labeling and was exploited to calculate the kinetics of photosynthetic 13CO2 uptake and to extrapolate the exact value of the 13CO2 peak concentration in the labeling chamber. Further, the leaf dark respiration of immature and mature leaves was analyzed. The quantification of the photosynthetic O2 production rate as a byproduct of the 13CO2 uptake correlated with the amount of available light and the leaf area of the plants in the labeling chamber. The ability to acquire CO2 and O2 respiration rates simultaneously also simplifies the determination of respiratory quotients (rate of O2 uptake compared to CO2 release) and thus indicates the type of combusted substrate. By combining quantification of respiration quotients with the tracing of 13C in plants, cavity enhanced Raman spectroscopy adds a valuable new tool for studies of metabolism at the organismal to ecosystem scale.
Introduction
Isotope labeling with gaseous precursors is an important tool in ecophysiological studies as it allows for a detailed investigation of the flow of resources at the level of an individual organism up to an entire ecosystem. For example, by labeling with 13C, the existence of different carbon allocation patterns between plant functional groups1 and the fast transfer of recently assimilated carbon to soil microorganisms2 have been demonstrated. 13C labeling has also been used at a molecular level to understand plant investments in secondary metabolites that serve as antiherbivore defenses3 as well as the biosynthetic pathways leading to defense-related compounds.4 Chemical ecology studies have monitored photosynthetic uptake of 13CO2 in real time in order to measure the incorporation of newly assimilated 13C into primary versus secondary metabolites under simulated herbivore pressure4,5 to address the growth-defense hypothesis.6 Measuring gas exchange of both 13CO2 and 12CO2 is important for investigations of the balance of the amount of incorporated 13C versus the amount of respirationally released 13CO2. 13C labeling of plants via exposure to pulses of 13CO2 is becoming a more commonly employed tool in studies of plant physiology and chemical ecology.7Nowadays, in most plant respiration experiments, CO2 production is measured as the sole parameter, either electrochemically or by non-dispersive infrared absorption spectroscopy.8 However, O2 consumption is also important for the determination of the respiratory quotient in order to draw conclusions about the type of combusted substrate and for quantification of the amount of label that got fixed by the plant. Most commonly used methods are not sufficiently sensitive in the measurement of O2 in plant respiration over timescales of minutes. High sensitivity O2 measurements are currently performed by taking gas samples for successive lab-based analyses using gas chromatography (GC) in combination with mass spectrometry (GCMS) in order to determine O2/N2 ratios. Unfortunately, these chromatographic techniques are slow, consumptive, and expensive, because the samples consist of complex mixtures of gases at various concentrations and several expensive test gases are needed for instrument calibration. To date, the miniaturization of test equipment for rapid online monitoring of multigas-samples (consisting of O2, N2, 13CO2 and 12CO2) has been limited.
These limitations can be circumvented by Raman spectroscopy, which provides characteristic information about molecular vibrations9 and thus chemical specificity. Raman spectroscopy emerged in recent years as an extremely powerful method10 in various natural science disciplines11 to investigate solid samples, liquids, and gases. Raman gas analysis is capable of quantifying almost all gases (except noble gases) simultaneously with just one measurement.12 This work introduces the methodology of cavity enhanced Raman multigas spectroscopy for pulse labeling studies of plant physiology.
Experimental details
Monoclonal cuttings of Populus trichocarpa were obtained from the “Thüringer Landesanstalt für Landwirtschaft”, Dornburg (Germany). Populus trichocarpa is a fast-growing species native to western North America. Cuttings were individually planted in 2 l pots with potting soil (Klasmann KKS Bio Topfsubstrat 27) mixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with quartz sand and grown in the greenhouse with additional light (Son-T Agro 430 W HPS bulbs, primary light range 520–610 nm, Philips Lighting Company, New Jersey, USA) from 6:00–17:00. The pots were uniformly watered with an irrigation system that delivered water two to three times for 3 min between 12:00 and 13:00 each day, depending on the temperature. The plants were moved to a climate chamber and exposed to five days of gradual cooling followed by an artificial winter of 8 h 10 °C days and 4 °C nights to induce senescence and leaf fall. The plants were returned to the greenhouse and exposed to the previous light and water conditions. As leaves were flushing, plants were exposed to 13CO2 in a 2 m3 labeling chamber (see Fig. 2). The gas phase was homogenized with the help of fans. Twenty-three to 25 plants were labeled on two consecutive days (runs 1 and 2, see Table 1) for approximately 2 h from 12:00 to 14:00. 13CO2 was introduced into the chamber via acidification of 2.67 g followed by 1.33 g 99% NaH13CO3 (Cambridge Isotope Laboratories, USA) with 16 ml or 8 ml diluted hydrochloric acid. The leaf area of every plant was measured at the time of labeling.
1 with quartz sand and grown in the greenhouse with additional light (Son-T Agro 430 W HPS bulbs, primary light range 520–610 nm, Philips Lighting Company, New Jersey, USA) from 6:00–17:00. The pots were uniformly watered with an irrigation system that delivered water two to three times for 3 min between 12:00 and 13:00 each day, depending on the temperature. The plants were moved to a climate chamber and exposed to five days of gradual cooling followed by an artificial winter of 8 h 10 °C days and 4 °C nights to induce senescence and leaf fall. The plants were returned to the greenhouse and exposed to the previous light and water conditions. As leaves were flushing, plants were exposed to 13CO2 in a 2 m3 labeling chamber (see Fig. 2). The gas phase was homogenized with the help of fans. Twenty-three to 25 plants were labeled on two consecutive days (runs 1 and 2, see Table 1) for approximately 2 h from 12:00 to 14:00. 13CO2 was introduced into the chamber via acidification of 2.67 g followed by 1.33 g 99% NaH13CO3 (Cambridge Isotope Laboratories, USA) with 16 ml or 8 ml diluted hydrochloric acid. The leaf area of every plant was measured at the time of labeling.
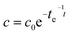
| Labeling run | O2 production [mol d−1] | Light intensity PAR [W m−2] | Total leaf area [m2] | 13CO2-uptake 1st dose te [min] | 13CO2-uptake 2nd dose te [min] |
|---|---|---|---|---|---|
| 1 | 0.35 | 121 | 2.29 | 23.52 | 20.6 |
| 2 | 0.38 | 275 | 3.11 | 17.16 | 15.7 |
Results and discussion
Raman gas monitoring of multigas mixtures containing isotopic labeled gases
The Raman gas sensor consists of a miniaturized laser diode with λexc. = 650 nm and a cw output power of 50 mW. This diode is passively frequency locked and feedback-coupled to a high-finesse cavity (PCB) enabling a power build-up to 100 W. Thus a strong signal enhancement is achieved with only low power consumption of the instrument. The PCB supports a Gaussian beam and consists of an input coupler mirror and an end mirror, both with extremely low scattering losses and transmission. For optimal beam enhancement and stable operation, the cavity components are aligned for spatial mode matching of the input beam and the Gaussian beam supported by the PBC while the facet of the laser diode helps in stabilizing mode matching by spatial filtering.13 This arrangement of the PCB is extremely stable to mechanical vibrations and is connected to a high-throughput spectrometer with a room temperature operated charge coupled device (CCD) with 512 pixels and a spectral resolution of approx. 50 cm−1. Additional sensors monitor the laser intensity, pressure and temperature for reliable gas quantification. With the help of this strong signal enhancement it was possible to monitor concentration fluctuations of about 50 to 100 ppm within measurement times of one second. The device was calibrated for the relevant gases, N2, O2, 12CO2, and 13CO2, by flushing the optical cavity with pure gases. Underground correction was accomplished by subtracting the spectrum of the Raman inactive noble gas argon. 13CO2 was calibrated with a GCMS-validated 1% mixture of 13CO2 with 99% argon (Raman inactive noble gas). The calibrated reference spectra are a prerequisite for the online quantification of sample gases during the isotope labeling experiment (Fig. 1). A straightforward calibration approach is feasible because the Raman intensity, IStokes, scales strictly linearly with the gas density in molecules per volume, nV−1, laser power, I0, and partial pressure, p over the whole dynamic range. | (1) |
Next, a least square fit of the measured spectrum and the calibrated reference spectra provided the concentrations of the individual gases. Therefore an over-determined linear equation system was solved with the calibration gas, g, measured gas, a (mixture of gases), intensity, I(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), concentration, c, CCD pixel, n, and number, m, of extracted gases.
), concentration, c, CCD pixel, n, and number, m, of extracted gases.
 | (2) |
Another major advantage of Raman multigas sensing becomes obvious from eqn (2), namely that all gases which appear in course of a labeling experiment will be detected in the Raman spectrum of the multigas-mixture. Thus, if the difference between the experimental multigas-spectrum and the deconvoluted individual spectra differs from a zero-baseline, more information about additional gases can be obtained by data post-processing with an increased number, m, of extracted gases. An example spectrum of an experimental gas mixture and the spectra of the gaseous components (N2, O2, and CO2) are depicted in Fig. 1A. The Raman gas spectra of the chemically similar gaseous isotopes, 12CO2 and 13CO2, can be readily distinguished due to their spectral shift and differences in the intensity pattern of the Fermi diad14 (Fig. 1B). Thus, all relevant gases (N2, O2, 12CO2, and 13CO2) can be quantified individually and simultaneously with no cross-sensitivity. The gas concentrations (N2, O2, 12CO2, 13CO2) obtained were normalized for a constant sum of all gases and a baseline subtraction was done for 12CO2 and 13CO2.
13CO2 labeling experiment of Populus trichocarpa
The utility of the new multigas sensing methodology is demonstrated in a labeling experiment that was designed to investigate the allocation of newly assimilated carbon to secondary metabolites. Saplings of Populus trichocarpa (see Experimental details) were exposed to 13CO2 in the chamber during a two hour pulse labeling experiment (Fig. 2). The Raman gas sensor was connected to the labeling chamber on the opposite side of the 13CO2 input to measure the labeled gas after it traveled through the chamber (Fig. 2). The Raman sensor always analyzed the homogenized gas concentration of the labeling chamber. Successive addition of the label was applied to increase the levels of 13C that could be incorporated into plant metabolites. Raman gas monitoring was applied to observe the maximum 13CO2 concentration and to ensure that the plants took up all the labeled 13C. The continuous online quantification of the 13CO2 level in the chamber allowed for accurate monitoring of the uptake during the labeling period and the precise timing of the second dose of 13CO2 (Fig. 3).First, the labeling chamber was flushed with CO2-free air to decrease the amount of 12CO2 from 586 ppm to <100 ppm within 0.5 h. At 0.7 h, 13CO2 was chemically generated from 13C-bicarbonate and diluted hydrochloric acid. A few minutes later the concentration of 13CO2 reached its maximum at 298 ppm and then decreased to <100 ppm after 1.6 h due to photosynthetic uptake by the plants (Fig. 3). After the complete uptake of the first dose, a second dose was administered. The 13CO2 concentration reached 182 ppm at 1.8 h and dropped to <100 ppm during the next half hour. At 2.75 h the labeling chamber was opened to ambient air. The concentration of O2 (calculated by a linear fit) rose by approximately 400 ppm during the labeling period, whereas the concentration of N2 did not change significantly.
A major drawback of conventional gas sampling techniques is the extended time needed for data analysis and consequently the small number of data points. In contrast, the high time resolution of Raman gas sensing was exploited for the rapid acquisition of many data points tracking the 13CO2-concentration during the course of the labeling, which allowed for kinetic investigations. First-order exponential fitting enabled the very precise determination of time constants (Table 1) and peak concentrations of 13CO2 (Table 2).
| Labeling run | 1st dose calculated [ppm] | 1st dose extrapolated [ppm] | 2nd dose calculated [ppm] | 2nd dose extrapolated [ppm] |
|---|---|---|---|---|
| 1 | 400 | 386 | 200 | 212 |
| 2 | 400 | 381 | 200 | 215 |
Two separate labeling runs were performed on different days, and more leaf area and photosynthetically active radiation (PAR) were available inside the labeling chamber in the second run. The comparison of both runs revealed that the amount of O2 produced during the 13CO2 labeling period was higher (0.38 mol per day compared to 0.35 mol per day) in run two. Similarly, the uptake of 13CO2 over time was faster in the second run, with a decay time of 15.7 min to reduce the amount of 13CO2 to 1/e of its starting value in comparison to 20.6 min in the first labeling run. All values are summarized in Table 1.
The high time resolution of Raman gas monitoring (Fig. 3) enabled the detection of small deviations from the exponential decay due to fluctuations in natural light intensity, and, by comparison of both doses, it was even possible to confirm that 13CO2 uptake by P. trichocarpa was faster at higher concentrations of 13CO2 within the course of the first dose because the photosynthesis rate of C3 plants is not strictly linear at low concentrations.15
An important task in environmental labeling experiments is the correct estimation of the peak concentration of 13CO2 in the labeling chamber. Peak concentrations are conventionally calculated based on the mass of the 13C-bicarbonate used to create the 13CO2. However, these approximations overestimate the value in the homogenized chamber atmosphere, due to the immediate photosynthetic 13CO2-uptake by the plants in the labeling chamber and the time for the chemical release of 13CO2 which broadens the sharpness of the labeling pulse. It is more precise to measure the 13CO2-concentration in the labeling chamber online and with rapid data acquisition by means of Raman gas sensing and calculate the peak concentration from the decay equation with high precision by extrapolating back to the time of the dose (Table 2). These peak concentrations of 13CO2 were lower with the first addition of 13CO2 than those calculated based on the mass of reacted bicarbonate, but the extrapolated values were higher after the second addition of 13CO2 in each labeling run (Table 2). This demonstrates that residual 13CO2 from the first dose was still present at the addition of the second dose, meaning actual 13CO2 values were noticeably higher than expected by standard bicarbonate-weight based calculations (Table 2).
In general, the amount of available data points from the temporally highly resolved Raman spectroscopic gas measurements enabled a very reliable fitting of time dependency curvatures and was well suited for kinetic investigations.
Leaf dark respiration
The labeled plants were further investigated with leaf dark respiration measurements in order to understand 13CO2 exchange over time. Approximately 24 h after the 13C-labeling, P. trichocarpa leaves of a known area were enclosed in a dark chamber, and the concentrations of O2, 12CO2, and 13CO2 were continuously recorded. The fluxes of all gases were calculated based on changes of the gas concentrations over time in the enclosed headspace. The fluxes were related to a leaf area that was based on one side of the leaf only to represent the area where the stomata are located. The respired gases were circulated through the instrument and returned to the chamber without consumption or alteration by the measurement. Ambient air was measured after one hour to validate the stability of experimental setting. A linear fit of the gas concentrations over time yielded rates for the uptake of O2 and CO2 production (Fig. 4). The current detection limit of the device for 12CO2 was 48 ppm (σ = 3) which results in a limit of detectable exchange rate of 0.12 μmol m−2 h−1. The additional 13CO2 efflux rate added by the highly diluted label from the plant was below this level. The leaf dark respiration measurements resulted in an O2 consumption rate of 1.81 μmol m−2 h−1 and a CO2 production rate of 1.80 μmol m−2 h−1 for immature leaves. In comparison, mature leaves consumed only 0.70 μmol m−2 h−1 of O2 and produced just 0.74 μmol m−2 h−1 of CO2. These results indicate that the respiration rates of immature leaves are more than twice as high as the respiration rates of mature leaves. Distinctly higher dark respiration rates of immature leaves are also reported in the literature.16 Simultaneous Raman spectroscopic quantification of O2 and CO2 also allowed for the calculation of the respiratory quotient (RQ) which yields information about the compounds being metabolized and respired. Deviations in the respiratory quotient arise from differing carbon–oxygen ratios of substrates or the formation of byproducts.17 The RQ values were ∼1 for all leaves, indicating the combustion of starch.18Conclusions and outlook
This work demonstrates the unique capabilities of innovative cavity enhanced Raman gas monitoring for the control and analysis of 13CO2-labeling experiments. Enhanced Raman gas sensing is superior to conventional Raman gas spectroscopy, due to the strong power build up to 100 W within the cavity (while maintaining low instrument power consumption) and outperforms other gas sensing techniques for the rapid and simultaneous analysis of multiple gases in a labeling chamber while eliminating sample collections and delayed analyses. The technique is non-consumptive, such that the measurements can be performed in a closed cycle with the labeling chamber without altering the gas composition. The high time resolution of the Raman measurement enables the acquisition of a huge number of data points, which tremendously increases the accuracy of kinetic investigations. Thus, it was possible to determine precise uptake rates and peak concentrations of 13CO2 in the pulse labeling of P. trichocarpa. Additionally, the simultaneous measurements of CO2 and O2 allowed for calculation of photosynthetic rates for both gases at once which correlated with the leaf area as well as the photosynthetically active radiation inside the labeling chamber. The investigation of leaf dark respiration of P. trichocarpa revealed that the respiration rate of immature leaves was more than twice as high compared to mature leaves. Simultaneous Raman gas quantification of O2 and CO2 enabled the calculation of the respiratory quotient, which is an indicator of the chemistry of metabolites that are fueling respiration, or can indicate the net effects of gas transport via the plant transpiration stream. Monitoring of 13CO2, 12CO2, and O2 also allows for quantification of the amount of label that got fixed by the plant and the 13C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12C-ratio.
12C-ratio.
Cavity enhanced Raman multigas sensing was shown to be a very versatile new technique for monitoring the amount of label incorporation in 13CO2-labeling experiments and it is also capable of rapidly analyzing the respiration quotient, an ecophysiologically important parameter. This new technique is affordable and very robust due to the linearity of signal intensity to analyte concentration. We therefore anticipate that cavity enhanced Raman spectroscopy (CERS) will become an important tool for labeling experiments in environmental research.
Acknowledgements
Funding of the research project by the Collaborative Research Centre 1076 “AquaDiva” from the Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged. The authors thank Dr. Willi Brand (MPI-BGC) for the calibration gases.References
- M. S. Carbone and S. E. Trumbore, Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns, New Phytol., 2007, 176(1), 124–135 CrossRef CAS PubMed.
- P. Högberg, M. N. Högberg, S. G. Göttlicher, N. R. Betson, S. G. Keel, D. B. Metcalfe, C. Campbell, A. Schindlbacher, V. Hurry, T. Lundmark, S. Linder and T. Näsholm, High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms, New Phytol., 2008, 177(1), 220–228 Search PubMed.
- T. Arnold and J. Schultz, Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus, Oecologia, 2002, 130(4), 585–593 CrossRef.
- E. C. Connor, A. S. Rott, M. Zeder, F. Juttner and S. Dorn, 13C-labelling patterns of green leaf volatiles indicating different dynamics of precursors in Brassica leaves, Phytochemistry, 2008, 69(6), 1304–1312 CrossRef CAS PubMed.
- D. Yakir and L. d. S. L. Sternberg, The use of stable isotopes to study ecosystem gas exchange, Oecologia, 2000, 123(3), 297–311 CrossRef.
- D. A. Herms and W. J. Mattson, The Dilemma of Plants: To Grow or Defend, Q. Rev. Biol., 1992, 67(3), 283 Search PubMed.
- D. Epron, M. Bahn, D. Derrien, F. A. Lattanzi, J. Pumpanen, A. Gessler, P. Hogberg, P. Maillard, M. Dannoura, D. Gerant and N. Buchmann, Pulse-labelling trees to study carbon allocation dynamics: a review of methods, current knowledge and future prospects, Tree Physiol., 2012, 32(6), 776–798 CrossRef CAS PubMed.
- (a) A. Saveyn, K. Steppe, M. A. McGuire, R. Lemeur and R. O. Teskey, Stem respiration and carbon dioxide efflux of young Populus deltoides trees in relation to temperature and xylem carbon dioxide concentration, Oecologia, 2008, 154(4), 637–649 CrossRef PubMed; (b) S. Hunt, Measurements of photosynthesis and respiration in plants, Physiol. Plant., 2003, 117(3), 314–325 CrossRef CAS.
- (a) T. Frosch, S. Koncarevic, K. Becker and J. Popp, Morphology-sensitive Raman modes of the malaria pigment hemozoin, Analyst, 2009, 134(6), 1126–1132 RSC; (b) T. Frosch and J. Popp, Structural analysis of the antimalarial drug halofantrine by means of Raman spectroscopy and density functional theory calculations, J. Biomed. Opt., 2010, 15(4), 041516 CrossRef PubMed; (c) T. Frosch, M. Schmitt, G. Bringmann, W. Kiefer and J. Popp, Structural analysis of the anti-malaria active agent chloroquine under physiological conditions, J. Phys. Chem. B, 2007, 111(7), 1815–1822 CrossRef CAS PubMed; (d) T. Frosch, M. Schmitt, K. Schenzel, J. H. Faber, G. Bringmann, W. Kiefer and J. Popp, In vivo localization and identification of the antiplasmodial alkaloid dioncophylline A in the tropical liana Triphyophyllum peltatum by a combination of fluorescence, near infrared Fourier transform Raman microscopy, and density functional theory calcula, Biopolymers, 2006, 82(4), 295–300 CrossRef CAS PubMed.
- (a) T. Frosch, T. Meyer, M. Schmitt and J. Popp, Device for Raman difference spectroscopy, Anal. Chem., 2007, 79(16), 6159–6166 CrossRef CAS PubMed; (b) T. Frosch, M. Schmitt, T. Noll, G. Bringmann, K. Schenzel and J. Popp, Ultrasensitive in situ tracing of the alkaloid dioncophylline A in the tropical liana Triphyophyllum peltatum by applying deep-UV resonance Raman microscopy, Anal. Chem., 2007, 79(3), 986–993 CrossRef CAS PubMed; (c) T. Frosch, N. Tarcea, M. Schmitt, H. Thiele, F. Langenhorst and J. Popp, UV Raman Imaging - A Promising Tool for Astrobiology: Comparative Raman Studies with Different Excitation Wavelengths on SNC Martian Meteorites, Anal. Chem., 2007, 79(3), 1101–1108 CrossRef CAS PubMed; (d) T. Frosch, D. Yan and J. Popp, Ultrasensitive Fiber Enhanced UV Resonance Raman Sensing of Drugs, Anal. Chem., 2013, 85(13), 6264–6271 CrossRef CAS PubMed.
- (a) T. Frosch, S. Koncarevic, L. Zedler, M. Schmitt, K. Schenzel, K. Becker and J. Popp, In situ localization and structural analysis of the malaria pigment hemozoin, J. Phys. Chem. B, 2007, 111(37), 11047–11056 CrossRef CAS PubMed; (b) T. Frosch, B. Küstner, S. Schlücker, a. Szeghalmi, M. Schmitt, W. Kiefer and J. Popp, In vitro polarization-resolved resonance Raman studies of the interaction of hematin with the antimalarial drug chloroquine, J. Raman Spectrosc., 2004, 35(10), 819–821 CrossRef CAS; (c) T. Frosch and J. Popp, Relationship between molecular structure and Raman spectra of quinolines, J. Mol. Struct., 2009, 924–926, 301–308 CrossRef CAS PubMed; (d) T. Frosch, M. Schmitt and J. Popp, In situ UV resonance Raman micro-spectroscopic localization of the antimalarial quinine in cinchona bark, J. Phys. Chem. B, 2007, 111(16), 4171–4177 CrossRef CAS PubMed; (e) T. Frosch, M. Schmitt and J. Popp, Raman spectroscopic investigation of the antimalarial agent mefloquine, Anal. Bioanal. Chem., 2007, 387(5), 1749–1757 CrossRef CAS PubMed.
- (a) T. Frosch, R. Keiner, B. Michalzik, B. Fischer and J. Popp, Investigation of gas exchange processes in peat bog ecosystems by means of innovative Raman gas spectroscopy, Anal. Chem., 2013, 85(3), 1295–1299 CrossRef CAS PubMed; (b) R. Keiner, T. Frosch, S. Hanf, A. Rusznyak, D. M. Akob, K. Kusel and J. Popp, Raman Spectroscopy-An Innovative and Versatile Tool To Follow the Respirational Activity and Carbonate Biomineralization of Important Cave Bacteria, Anal. Chem., 2013 Search PubMed; (c) R. Salter, J. Chu and M. Hippler, Cavity-enhanced Raman spectroscopy with optical feedback cw diode lasers for gas phase analysis and spectroscopy, Analyst, 2012, 137(20), 4669–4676 RSC.
- D. A. King and R. J. Pittaro, Simple diode pumping of a power-build up cavity, Opt. Lett., 1998, 23(10), 774–776 CrossRef CAS.
- H. Howardlock and B. Stoicheff, Raman intensity measurements of the Fermi diad ν1, 2ν2 in 12CO2 and 13CO2, J. Mol. Spectrosc., 1971, 37(2), 321–326 CrossRef CAS.
- T. D. Sharkey, Photosynthesis in intact leaves of C3 plants: Physics, physiology and rate limitations, Bot. Rev., 1985, 51(1), 53–105 CrossRef.
- D. I. Dickmann, Photosynthesis and Respiration by Developing Leaves of Cottonwood (Populus deltoides Bartr.), Bot. Gaz., 1971, 132(4), 253–259 Search PubMed.
- J. Azcon-Bieto, H. Lambers and D. A. Day, Effect of Photosynthesis and Carbohydrate Status on Respiratory Rates and the Involvement of the Alternative Pathway in Leaf Respiration, Plant Physiol., 1983, 72(3), 598–603 CrossRef CAS PubMed.
- N. G. McDowell, Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality, Plant Physiol., 2011, 155(3), 1051–1059 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |




