Facile synthesis of single-crystalline hollow α-Fe2O3 nanospheres with gas sensing properties†
Pei-Pei Wanga,
Xiaoxin Zoua,
Liang-Liang Fenga,
Jun Zhaoa,
Pan-Pan Jina,
Rui-Fei Xuanb,
Ye Tianc,
Guo-Dong Lia and
Yong-Cun Zou*a
aState Key Lab of Inorganic Synthesis & Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: zouyc@jlu.edu.cn; Fax: +86-431-85168624; Tel: +86-431-85168318
bCollege of Materials Science and Engineering, China University of Mining and Technology, Xuzhou 221116, China
cTianjin Key Laboratory of Applied Catalysis Science and Technology, School of Chemical Engineering, Tianjin University, Tianjin 300072, China
First published on 13th August 2014
Abstract
High-quality single-crystalline hollow α-Fe2O3 nanospheres were prepared, using the ZnS–CHA (CHA = cyclohexylamine) nanohybrid as an additive through a solvothermal reaction, which avoids tedious steps and a high temperature calcination process. The formation process of these hollow nanospheres can be divided into two stages: (i) formation of solid Fe2O3 nanospheres and (ii) preferential inside-out dissolution of the solid nanoparticles to form hollow nanospheres. Due to the unique single-crystalline hollow structure, the as-obtained α-Fe2O3 nanomaterial exhibits enhanced gas sensing properties.
As an important n-type semiconductor, α-Fe2O3 has been widely studied for its comprehensive applications such as for gas sensors, Li-ion batteries, pigments, magnetic recorders and catalysts.1–11 Considerable research efforts have been devoted to develop methods for delicate control over the morphology, size and functions of α-Fe2O3. Most of the reported work evolves around methodologies of constructing hollow or single-crystalline Fe2O3,2,5,12–19 among which single-crystalline hollow Fe2O3 was the best representative.17,20 The first single-crystalline hollow Fe2O3 was reported by Eswaramoorthy's group, using carbonaceous spheres as sacrificial templates with a sol–gel process. The single-crystalline hollow spheres were homogeneous, but they were collected by high temperature post-calcination which was a bit energy consumption.5 Fan and his coworkers reported a shape-controlled method making hollow single-crystalline Fe2O3 by hydrothermal treatment at 220 °C for 60 h, which process was easy to realize but a bit time-consuming.21 Combining the functionality of Fe2O3 with its single-crystalline hollow structure is of essential for function optimum. Quest for simple, controllable and scalable synthesis of single-crystalline hollow Fe2O3 materials have been obtained tremendous attention.
In this paper, we report a facile template-free approach to prepare single-crystalline hollow Fe2O3 with ZnS–CHA nanohybrid as an additive. The procedure was conducted without post-high temperature calcination process within 24 h. The hollow nanospheres are in diameters of 200–300 nm with well-defined shape and size, and the size of the shells are around 20–50 nm. And the as-obtained Fe2O3 exhibits promising chemical sensing properties. The hollow single-crystalline α-Fe2O3 nanospheres show good gas sensing property towards ethanol, and exhibits good response characteristics towards multiple of target gases. As the raw material, ZnS–CHA nanocomposite, was easy to sale up, the current contribution may offer a versatile approach for the development of a series of metal oxide for advanced applications.
Fig. 1A shows the SEM image of the single-crystalline Fe2O3 with hollow spheres in uniformed size, and the hollow structure was further determined by TEM analysis, which exhibits the diameter in the range of 200–300 nm with wall thickness around 20–50 nm (Fig. 1B). High resolution TEM (HRTEM) show the particles with continuous lattice fringes measured from (101) plane to be 0.42 nm and (006) plane to be 0.23 nm in (Fig. 1C), and the selected area electron diffraction (SAED) pattern (Fig. 1D) evidently gives the single-crystalline nature of the nanospheres.
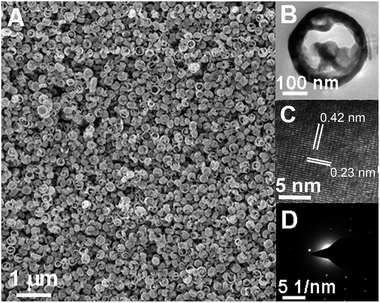 | ||
| Fig. 1 (A) SEM; (B) TEM; (C) HRTEM images and (D) SAED pattern of the obtained single-crystalline α-Fe2O3 nanospheres. | ||
High-quality single-crystalline hollow α-Fe2O3 nanospheres were prepared using ZnS–CHA nanohybrid as an additive. In the typical system, zinc acetate dihydrate and thiourea functioned as the zinc source and the sulfur source, respectively, whereas cyclohexylamine (CHA) acted as both the solvent and the reactive agent. In comparison with the previous synthetic condition of ZnS–CHA, we just decreased the concentration of the zinc source (75 mmol L−1 in referenced paper and 37.5 mmol L−1 in this paper) in the reaction mixture to get a high-quality ZnS–CHA sample, while maintaining the molar ratio of zinc to sulfur (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The structure of as-prepared ZnS–CHA was similar to that in the previous report, as demonstrated by powder X-ray diffraction (XRD), infrared spectroscopy (IR), and X-ray photoelectron spectroscopy (XPS) (see Fig. S1 in the ESI†). The ZnS–CHA nanocomposite was formed from the very small ZnS nanoparticles through assembly with the CHA molecules. The zinc and sulfur species were Zn2+ and S2−, respectively, and the CHA molecules were not protonated in the ZnS–CHA nanocomposite. Based on the thermogravimetric (TG) result (Fig. S1C†), the empirical composition of the as-prepared ZnS–CHA nanocomposite was close to ZnS·CHA, in which the amount of CHA was obviously higher than that reported previously, probably due to the higher CHA
2). The structure of as-prepared ZnS–CHA was similar to that in the previous report, as demonstrated by powder X-ray diffraction (XRD), infrared spectroscopy (IR), and X-ray photoelectron spectroscopy (XPS) (see Fig. S1 in the ESI†). The ZnS–CHA nanocomposite was formed from the very small ZnS nanoparticles through assembly with the CHA molecules. The zinc and sulfur species were Zn2+ and S2−, respectively, and the CHA molecules were not protonated in the ZnS–CHA nanocomposite. Based on the thermogravimetric (TG) result (Fig. S1C†), the empirical composition of the as-prepared ZnS–CHA nanocomposite was close to ZnS·CHA, in which the amount of CHA was obviously higher than that reported previously, probably due to the higher CHA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio in this reaction condition. In addition, the morphology of the as-prepared ZnS–CHA with a short rod-shape (see SEM image in Fig. S2†) was also different from that reported previously (irregular powder sample).22 It should be noted that, the exact crystal structure was still not available since the large single crystal of ZnS–CHA can't be obtained. Regardless of its exact crystal structure, ZnS–CHA has been applied as an efficient precursor for the preparation of inorganic nanosheet material.23 It was also used as a novel additive for formation of the well-defined α-Fe2O3 nanospheres, as demonstrated as below.
Zn ratio in this reaction condition. In addition, the morphology of the as-prepared ZnS–CHA with a short rod-shape (see SEM image in Fig. S2†) was also different from that reported previously (irregular powder sample).22 It should be noted that, the exact crystal structure was still not available since the large single crystal of ZnS–CHA can't be obtained. Regardless of its exact crystal structure, ZnS–CHA has been applied as an efficient precursor for the preparation of inorganic nanosheet material.23 It was also used as a novel additive for formation of the well-defined α-Fe2O3 nanospheres, as demonstrated as below.
In order to understand the formation process of the uniform hollow α-Fe2O3, time-dependent experiments were carried out carefully on basis of the above experimental procedures. At given time interval, the powder products were harvested by centrifuging and washing with deionized water for further characterization. Corresponding SEM photographs were shown in Fig. S3.† As shown in the pictures, the nanoparticles were so small that we couldn't see them clearly during 1 h (Fig. S3A†). After that, the solid products we collected were with diameters in the range of 200–300 nm (Fig. S3B and C†). It doesn't form the completely hollow nanospheres until 24 h later (Fig. S3E and F†), and the diameters of the nanospheres were not changed obviously. The schematic diagram for formation of the hollow nanosphere was shown in Scheme S1.† Based on the above results, the formation process of these hollow nanospheres can be mainly divided into two stages: (i) formation of solid Fe2O3 nanospheres and (ii) preferential inside-out dissolution of the solid nanoparticles to form hollow nanospheres.
| Fe3+ + H2O → Fe(OH)3/FeOOH + H+ | (1) |
| Fe(OH)3/FeOOH → Fe2O3 + H2O | (2) |
Under a hydrothermal reaction condition, the formation of Fe2O3 nanoparticles from Fe3+ ions is based on the eqn (1) and (2). Then the 3-D oriented attachment of nanocrystals was formed,4,5,24 the nanocrystals began to self-assembly well-defined single-crystalline α-Fe2O3 solid nanospheres. Accompanying this process, the pH value of the reaction system will decrease due to the generation of protons (eqn (1); the pH value of the final reaction system is as low as 2.4). This is will provide an acidic condition to dissolve the ZnS–CHA hybrid and to selectively inside-out dissolve the solid Fe2O3 nanospheres because the inner part was less stable compared with the outer surface layer of Fe2O3 nanocrystal.5,25 At last, the hollow single-crystalline nanospheres were formed.
XRD patterns were shown in Fig. 2A. It was clear that the product was consists of α-Fe2O3 and ZnS at 1 h, and the pure α-Fe2O3 (JCPDS card no.: 33-0664) with no impure peaks was formed after 6 h. The product we obtained after 24 h was in good crystalline with narrow peaks and smooth base line. Raman spectra was also carried out to determine the crystal phase and crystalline condition (Fig. 2B). The characteristics roman bands were typical α-Fe2O3. The base lines of the products in 1 h and 6 h were a bit rough revealing the low crystalline. At last, the hollow Fe2O3 in better quality crystalline was obtained after 24 h. The Raman spectra matched very well with the XRD patterns.
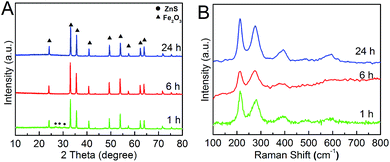 | ||
| Fig. 2 (A) XRD patterns and (B) Raman spectra of the α-Fe2O3 nanospheres obtained at 1 h, 6 h and 24 h. | ||
Surface elements of the hollow nanospheres were examined by XPS spectra shown in Fig. S4.† High-resolution of Fe 2p1/2 and Fe 2p3/2 peaks of the hollow α-Fe2O3 (Fig. S4A†) were located at 725.3 and 711.2 eV, respectively. The shakeup satellite at 719.9 eV was the typical Fe3+ in Fe2O3.4,8 High-resolution of O 1s was shown in Fig. S4B.† The vibrations of crystal lattice O occupied nearly 82.8%, and the adsorption of molecule oxygen takes only 27.2%.
In order to determine the role of ZnS–CHA nanohybrid in formation of the well-defined single-crystalline hollow α-Fe2O3, four different control experiments were performed for comparative studies. (1) The first control experiment involved the synthesis of materials with no ZnS–CHA being used as the above synthetic procedure, this resulted in only uneven α-Fe2O3 nanoparticles formed with average size of ∼300 nm (Fig. S5A†). The product we collected in this process was called Fe2O3-1; (2) instead of ZnS–CHA, the same amount of pure CHA (0.015 g) was used under identical reaction conditions. However, the synthesis also resulted in irregular α-Fe2O3 nanoparticles with average size of ∼100 nm (Fig. S5B†) which was called Fe2O3-2; (3) treating the ZnS–CHA nanocomposite at 160 °C in water solution to synthesis the pure ZnS, and then taking the same amount of ZnS instead of the ZnS–CHA nanohybrid, the final product was also uneven α-Fe2O3 nanoparticles with average size of ∼300 nm (Fig. S5C†) which was called Fe2O3-3; (4) the as prepared pure ZnS (0.015 g) with pure CHA (0.015 g) were used to react at the same condition as mentioned above, without exception, the final product was also uneven nanoparticles with average size of ∼100 nm (Fig. S5D†) named as Fe2O3-4. Therefore, we can conclude that the ZnS–CHA nanohybrid plays an essential role in preparation of the well-defined single-crystalline hollow α-Fe2O3 nanospheres.
To further confirm the relationship between the iron content with the single-crystalline hollow Fe2O3, controlled experiments were conducted by using 0.32 g, 0.48 g and 0.64 g FeCl3·6H2O. As a result, the obtained Fe2O3 nanospheres were polycrystalline with increased diameters and the corresponding SEM images were given in Fig. S6.†
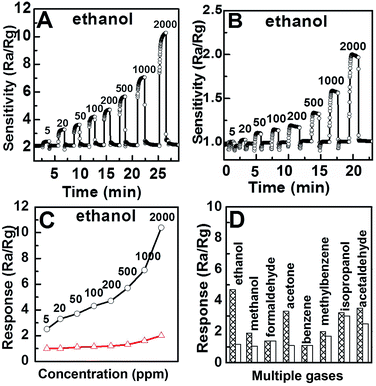
Gas sensing performance was employed by a CGS-8 gas sensing measurement system (Beijing Elite Tech Company Limited). The whole process was the same as mentioned before.26–28 Sensitivity was designed as Ra/Rg, where Ra was the resistance of sensors in air, Rg was the resistance of the sensors in the target gases.9,29,30 Gas sensing properties of the single crystal hollow α-Fe2O3 nanospheres were researched towards ethanol. The concentration–sensitivity relationship experiments were executed from 5 ppm to 2000 ppm at 300 °C (Fig. 3A). The sensitivity was increased with increasing the concentration of ethanol. Excepting the sensitivity, there were also some vital parameters for the sensing materials, such as response time, recovery time and the stability. The magnification curve of the single-crystalline hollow Fe2O3 towards 500 ppm ethanol was shown in Fig. S9.† The response time was defined as the time from Ra to Ra − 90% × (Ra − Rg), and the recovery time was defined as the time from Rg to Rg + 90% × (Ra − Rg). The single-crystalline hollow Fe2O3 exhibits short response time for 5 s and fast recovery time for 2 s, both of which were much better than the reported results.16,31 The dynamic response curves of the contrast Fe2O3-1 mentioned above (control experiment (1)) to ethanol was shown in Fig. 3B. Concentration–response curves of the two sensors were shown in Fig. 3C. The single-crystalline hollow Fe2O3 shows the superior response towards ethanol compared to contrast Fe2O3-1 shown in Fig. 3D. The single-crystalline hollow Fe2O3 also gives higher response to other gases such as acetone, methanol, and formaldehyde than the contrast Fe2O3-1. In order to estimate the stability of the single-crystalline hollow Fe2O3, time depended gas sensing experiments were taken every one day for ten days. Slight variations were found during the ten days measurement towards 5, 500 and 2000 ppm ethanol (Fig. S10†). This results give the final conclusion that the sensors exhibit good repeatability to ethanol.
The sensing mechanism of Fe2O3 sensors was the same as the traditional semiconductor sensors. The main mechanism was based on the reaction between the detected gas molecules and the chemisorbed oxygen species on Fe2O3 surface. The Fe2O3 sensors will exhibits a relatively high resistance state by generate O2− or O− in the air condition,32 since the adsorbed oxygen molecules will capture electrons from the Fe2O3 layer (conduction band of the Fe2O3). When the target reductive gases were introduced at an moderate operating temperature (e.g., ethanol), the Fe2O3 surface layer oxygen species will have a reaction with them, and then the electrons will be given back into the Fe2O3 layer, giving rise to the reduced surface oxygen species along with the decreased surface resistance. The reaction equation was presented as follows:
| C2H5OH + 6O− ↔ 2CO2 + 3H2O + 6e− | (3) |
The enhanced sensing performance may attribute to the hollow structure of the single-crystalline Fe2O3. TEM and HRTEM images of Fe2O3-1 were shown in Fig. S7,† which further confirmed its solid structure. Single-crystalline hollow Fe2O3 owns larger BET surface area, the BET surface area of the single-crystalline hollow Fe2O3 was 31 m2 g−1 (Fig. S8A†), remarkably higher than that of the Fe2O3-1 (Fig. S8B,† 12 m2 g−1). The hollow structure with higher surface to volume ratio could provide more active sites for gas molecules adsorption, leading to the higher sensitivity and shorter response–recovery times. The sparse physiognomy make the single-crystalline hollow Fe2O3 nanospheres a good candidate for high performance sensing material.
Conclusion
Single-crystalline hollow hematite (Fe2O3) nanospheres were synthesized through template-free solvothermal reaction, without surfactant and high temperature calcination process. The diameters of the nanospheres were around 200–300 nm with uniform shape and size. The shells of the spheres were on the nanoscale around 20–50 nm. The ZnS–CHA nanohybrid, which used as the source material, plays a necessary role in preparation of the uniform single α-Fe2O3 nanospheres. The hollow nanospheres show good gas sensing property towards ethanol. This method provides us an easy and convenient way to synthesize single crystalline hollow nanospheres.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21371070); Science and Technology Development Projects of Jilin Province (20140101041JC, 20130204001GX); Natural Science Foundation of Tianjin, China (12JCYBJC14000).Notes and references
- H.-J. Song, X.-H. Jia, H. Qi, X.-F. Yang, H. Tang and C.-Y. Min, J. Mater. Chem., 2012, 22, 3508 RSC.
- B. Wang, J. S. Chen, H. B. Wu, Z. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146 CrossRef CAS PubMed.
- L. Xu, J. Xia, K. Wang, L. Wang, H. Li, H. Xu, L. Huang and M. He, Dalton Trans., 2013, 42, 6468 RSC.
- X. Hu, J. C. Yu, J. Gong, Q. Li and G. Li, Adv. Mater., 2007, 19, 2324 CrossRef CAS PubMed.
- D. Jagadeesan, U. Mansoori, P. Mandal, A. Sundaresan and M. Eswaramoorthy, Angew. Chem., Int. Ed., 2008, 47, 7685 CrossRef CAS PubMed.
- H.-J. Kim, K.-I. Choi, A. Pan, I.-D. Kim, H.-R. Kim, K.-M. Kim, C. W. Na, G. Cao and J.-H. Lee, J. Mater. Chem., 2011, 21, 6549 RSC.
- D. Chen, W. Wei, R. Wang, J. Zhu and L. Guo, New J. Chem., 2012, 36, 1589 RSC.
- T. Wang, S. Zhou, C. Zhang, J. Lian, Y. Liang and W. Yuan, New J. Chem., 2014, 38, 46 RSC.
- P. Sun, Z. Zhu, P. Zhao, X. Liang, Y. Sun, F. Liu and G. Lu, CrystEngComm, 2012, 14, 8335 RSC.
- N. V. Long, Y. Yang, M. Yuasa, C. M. Thi, Y. Cao, T. Nann and M. Nogami, RSC Adv., 2014, 4, 8250 RSC.
- L. Zhang, H. B. Wu, R. Xu and X. W. Lou, CrystEngComm, 2013, 15, 9332 RSC.
- M. Cao, T. Liu, S. Gao, G. Sun, X. Wu, C. Hu and Z. L. Wang, Angew. Chem., Int. Ed., 2005, 44, 4197 CrossRef CAS PubMed.
- H. Deng, X. Li, Q. Peng, X. Wang, J. Chen and Y. Li, Angew. Chem., 2005, 117, 2842 CrossRef PubMed.
- B. Lv, Z. Liu, H. Tian, Y. Xu, D. Wu and Y. Sun, Adv. Funct. Mater., 2010, 20, 3987 CrossRef CAS PubMed.
- X. Liu, J. Zhang, S. Wu, D. Yang, P. Liu, H. Zhang, S. Wang, X. Yao, G. Zhu and H. Zhao, RSC Adv., 2012, 2, 6178 RSC.
- X. Li, W. Wei, S. Wang, L. Kuai and B. Geng, Nanoscale, 2011, 3, 718 RSC.
- B. Lv, Y. Xu, D. Wu and Y. Sun, Chem. Commun., 2011, 47, 967 RSC.
- N. K. Chaudhari, H. Chan Kim, D. Son and J.-S. Yu, CrystEngComm, 2009, 11, 2264 RSC.
- S. Bharathi, D. Nataraj, M. Seetha, D. Mangalaraj, N. Ponpandian, Y. Masuda, K. Senthil and K. Yong, CrystEngComm, 2010, 12, 373 RSC.
- W. Wu, R. Hao, F. Liu, X. Su and Y. Hou, J. Mater. Chem. A, 2013, 1, 6888 CAS.
- H. Fan, G. You, Y. Li, Z. Zheng, H. Tan, Z. Shen, S. Tang and Y. Feng, J. Phys. Chem. C, 2009, 113, 9928 CAS.
- X. X. Zou, G. D. Li, J. Zhao, P. P. Wang, Y. N. Wang, L. J. Zhou, J. Su, L. Li and J. S. Chen, Inorg. Chem., 2011, 50, 9106 CrossRef CAS PubMed.
- Y. Yu, J. Zhang, X. Wu, W. Zhao and B. Zhang, Angew. Chem., Int. Ed., 2012, 51, 897 CrossRef CAS PubMed.
- L.-P. Zhu, H.-M. Xiao, W.-D. Zhang, G. Yang and S.-Y. Fu, Cryst. Growth Des., 2008, 8, 957 CAS.
- X. Wu, Y. Yu, Y. Liu, Y. Xu, C. Liu and B. Zhang, Angew. Chem., Int. Ed., 2012, 51, 1 CrossRef PubMed.
- P.-P. Wang, Q. Qi, X. Zou, J. Zhao, R.-F. Xuan and G.-D. Li, RSC Adv., 2013, 3, 23980 RSC.
- J. Zhong, C. Cao, Y. Liu, Y. Li and W. S. Khan, Chem. Commun., 2010, 46, 3869 RSC.
- J. Zhao, X. Zou, L.-J. Zhou, L.-L. Feng, P.-P. Jin, Y.-P. Liu and G.-D. Li, Dalton Trans., 2013, 42, 14357 RSC.
- C. Zhao, G. Zhang, W. Han, J. Fu, Y. He, Z. Zhang and E. Xie, CrystEngComm, 2013, 15, 6491 RSC.
- X.-X. Zou, G.-D. Li, P.-P. Wang, J. Su, J. Zhao, L.-J. Zhou, Y.-N. Wang and J.-S. Chen, Dalton Trans., 2012, 41, 9773 RSC.
- Q. Hao, S. Liu, X. Yin, Z. Du, M. Zhang, L. Li, Y. Wang, T. Wang and Q. Li, CrystEngComm, 2011, 13, 806 RSC.
- J. Y. Liu, Z. Guo, F. L. Meng, Y. Jia, T. Luo, M. Q. Li and J. H. Liu, Cryst. Growth Des., 2009, 9, 1716 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization details of the ZnS–CHA nanocomposite, SEM and TEM of the relevant Fe2O3 products. See DOI: 10.1039/c4ra05651e |
| This journal is © The Royal Society of Chemistry 2014 |