Poly-l-lysine assisted synthesis of core–shell nanoparticles and conjugation with triphenylphosphonium to target mitochondria†
Abstract
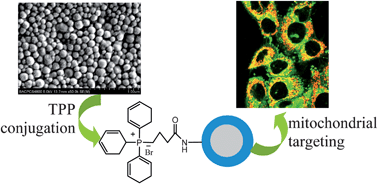
In this paper, we report a facile route to synthesize mitochondria-targeted core–shell nanoparticles (NPs). Firstly, PLL-coated NPs are prepared by a one-step reprecipitation-encapsulation method assisted by positively charged poly-L-lysine (PLL). The effect of the molecular weight of PLL on the formation of particles is studied in terms of morphology, size and zeta potential, and medium-sized PLL (MH-PLL) is proved to be the optimum one. By means of crosslinking with different amounts of glutaraldehyde, amino groups in MH-PLL-NPs are characterized by zeta potential and fluorescamine assay, respectively. The results indicate that in the PLL shell, only a small portion of amino groups (surface amino groups, SAGs) are available for conjugation, while the other groups exclusively contribute to zeta potential. Subsequently, a known mitochondriotropic ligand, triphenylphosphonium (TPP), is conjugated with SAG via a carbodiimide reaction, which is evaluated by NMR and absorption spectra, respectively. The TPP-MH-PLL-NPs exhibit a low cytotoxic effect tested by the MTT method, as well as efficient cellular uptake microscopically observed after a fluorescent dye, coumarin 6, is incorporated. Most importantly, the TPP-conjugated NPs can selectively target mitochondria, demonstrated by the merged z-stacked images in co-localization experiments with MitoTracker-stained mitochondria. Given that many hydrophobic species could be loaded into the particle core, TPP-MH-PLL-NPs are very promising as mitochondria-targeted nanocarriers for imaging or anti-cancer therapies.

 Please wait while we load your content...
Please wait while we load your content...