Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionothermal, microwave-assisted synthesis of indium(III) selenide
Sophie
Tyrrell
,
Małgorzata
Swadźba-Kwaśny
and
Peter
Nockemann
*
QUILL, The Queen's University of Belfast, Belfast, BT9 5AG, UK. E-mail: p.nockemann@qub.ac.uk
First published on 3rd December 2013
Abstract
Microcrystalline indium(III) selenide was prepared from a diphenyl diselenide precursor and a range of chloroindate(III) ionic liquids via a microwave-assisted ionothermal route; this is the first report on the use of either microwave irradiation or ionic liquids to prepare this material. The influence of the reaction temperature, dilution with a spectator ionic liquid and variation of the cation and the anion of the ionic liquid on the product morphology and composition were investigated. This resulted in a time-efficient and facile one-pot reaction to produce microcrystalline indium(III) selenide. The product formation in the ionic liquids has been monitored using Raman spectroscopy. The products have been characterised using PXRD, SEM and EDX. Advantages of this new route, such as the ease of solubilisation of all reactants into one phase at high concentration, the negligible vapour pressure irrespective of the reaction temperature, very fast reaction times, ease of potential scale-up and reproducibility are discussed.
Introduction
Indium(III) selenide is a black crystalline n-type, III–VI layered semiconductor with a direct band gap of 1.7 eV.1 The band gaps of III–VI layered semiconductors are generally narrow at room temperature, which makes them attractive compounds for solar energy conversion and optoelectronic devices2,3 and therefore, have potential in solar energy conversion applications.4 To date they have been studied as precursors to copper indium selenide, CIS, as an optical recording medium, a polarizer for optoelectronics and in Li-collated form for battery applications.5Structurally, In2Se3 comprises primitive layers of four atomic planes, Se–In–In–Se; the selenium atoms form two-dimensional hexagonally close-packed sheets, which provides the crystal with the hexagonal structure.5 The two most common forms of crystalline In2Se3 are the α (layered structure) and γ (the ‘defect wurtzite structure’);6 other commonly known forms include the β and δ phases,7 as well as the recently discovered κ phase (anisotropic structure).8 Depending on the synthetic technique and reaction conditions, one of the crystalline forms of indium(III) selenide is produced, each form having a unique range of electronic and structural properties. For example, α-In2Se3 has cation vacancies, which form a plane that results in weak Se–Se bonding and anisotropic electronic properties. In2Se3 is an anisotropic hexagonal or rhombohedral semiconductor that tends to have high resistivity. Importantly, when low reaction temperatures are implemented, indium selenide(II) crystals with a zinc blende structure are formed, while at higher temperatures hexagonal crystals of In2Se3 are obtained. In general, the presence of numerous forms of indium selenide, even in perfectly stoichiometric compositions, makes interpretation of exact product stoichiometry, electronic and optical data more difficult.8 The molar ratio of precursors and reaction temperature influence the type of indium(III) selenide produced, but not the shape and size of the product.9
Various atomic arrangements of indium(III) selenide materials have been reported in the literature: microporous (framework) materials of high structural complexity,10,11 open framework chalcogenides, noncluster-based 3D open-framework indium chalcogenides,12,13 with a range of morphologies also described, including, thin films,1,4 and nanomaterials.14 Traditional synthetic routes involve long reaction times (up to 10 days)43 with high temperatures, up to 450 °C,15 and high pressures, often combined with using hazardous organic solvents, such as ethylenediamine, hydrazine or 3,5-dimethylpyridine. These solvents are necessary due to the troublesome dissolution of elemental selenium, generally used as a precursor.16
In this contribution, we propose an innovative route to microcrystalline indium(III) selenide, taking advantage of possibilities offered by ionic liquid-based synthesis. Ionic liquids are salts, typically based on organic cations, with melting points below 100 °C, and often much lower than ambient temperature.17,18 Among their common characteristics are negligible vapour pressure and ability to dissolve a wide range of organic and inorganic materials,19–22 with a solubility significantly higher than that exhibited for most organic solvents.23 Ionic liquids have an extraordinarily high solubility for metal halides when placed in highly polar Lewis acidic systems, even at room temperature. This has allowed for a low temperature one-pot synthesis of a range of chalcogenide materials.24 The excellent solubility allows for synthesis of nano, micro and macro-sized inorganic products, with new materials being possible due to the change from molecular to ionic solvent systems.21 This has led to the development of a new synthetic approach based on ionic liquids, named ‘ionothermal’ synthesis,21,25,26 in analogy to hydrothermal processes. In a typical ionothermal process, all reactants are dissolved in an ionic liquid, which is subsequently heated to allow the reaction to proceed, with ambient pressure maintained due to the non-volatile nature of ionic liquids.
Chlorometallate ionic liquids are a class of ionic liquids based on an organic cation and a chlorometallate anion.27 They are prepared by the reaction of an organic chloride salt with a metal chloride; various ratios of reactants may be used, which leads to different anionic speciation, as exemplified for the In(III) system in eqn (1)–(3).28
| 1 [cation]Cl + InCl3 → [cation][InCl4] | (1) |
| 2 [cation]Cl + InCl3 → [cation]2[InCl5] | (2) |
| 3 [cation]Cl + InCl3 → [cation]3[InCl6] | (3) |
These anions remain in a dynamic equilibrium with each other, as shown in eqn (4).
| [InCl5]2− ⇄ [InCl4]− + [InCl6]3− | (4) |
Chlorometallate ionic liquids are excellent media for the synthesis of inorganic materials; besides ‘regular’ advantages of ionic liquids, they also act as a metal source, available in very high concentrations and with a tuneable coordination.29 To date, chlorometallate ionic liquids have been used in dual roles of solvents and precursors to prepare a range of inorganic materials: CuICl nanoplatelets,30 In0,31 Au0 and Pd0 nanoparticles32 and complete families of semiconductor materials, such as [Bi2Te2Br][AlCl4],33 [(Bi4Te4Br2)(Al2Cl(6−x)Brx)]Cl2 and [Bi2Se2Br][AlCl4].34 In many cases it is emphasised that the cation plays a significant role, acting as a templating and/or stabilising agent.30–32,35
Microwave-assisted synthesis may, in many cases, dramatically reduce the reaction time, and has been successfully implemented in the preparation of a variety of inorganic materials.36 Ionic liquids readily absorb microwave irradiation and do not increase vapour pressure upon rapid heating, hence are particularly suitable for fast-paced microwave-assisted synthesis.37
To our knowledge, neither ionic liquids and the ionothermal approach, nor microwave-based synthesis have been adopted to prepare indium(III) selenide. In this work, advantages of chlorometallate ionic liquids and microwave-assisted synthesis were combined to access microcrystalline indium(III) selenide in a fast, safe and elegant synthetic pathway.
Experimental
Materials
Indium(III) chloride, 99%, anhydrous, and diphenyl diselenide, 99%, and indium(III) selenide were purchased from Sigma-Aldrich. Ionic liquid families: alkyl(trioctyl)phosphonium chloride, [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n]Cl, and alkyl(trihexyl)phosphonium chloride, [P6
n]Cl, and alkyl(trihexyl)phosphonium chloride, [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n]Cl, (n = 3, 5, 10, 14, 17 and 18) were prepared as described elsewhere.38 1-Octyl-3-methylimidazolium chloride, [C8mim]Cl, was prepared as previously reported.28
n]Cl, (n = 3, 5, 10, 14, 17 and 18) were prepared as described elsewhere.38 1-Octyl-3-methylimidazolium chloride, [C8mim]Cl, was prepared as previously reported.28
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10]Cl (0.60 g, 1.097 mmol) was dissolved in 6 cm3 of dichloromethane. LiNTf2 (2.16 g, 7.526 mmol) was dissolved in 6 cm3 of water. Both solutions were poured into a round-bottomed flask (250 cm3) containing a stirring bar. The flask was closed with a stopper and the mixture was stirred vigorously overnight. Subsequently, the top (aqueous) layer was decanted, and the bottom (ionic liquid) layer was washed three times with an aqueous solution of LiNTf2 (0.02 mol cm−3) and ten times with deionised water. Remaining solvents were removed using a rotary evaporator (80 °C, 1 h) and then high vacuum (80 °C, overnight, 10−2 mbar).
10]Cl (0.60 g, 1.097 mmol) was dissolved in 6 cm3 of dichloromethane. LiNTf2 (2.16 g, 7.526 mmol) was dissolved in 6 cm3 of water. Both solutions were poured into a round-bottomed flask (250 cm3) containing a stirring bar. The flask was closed with a stopper and the mixture was stirred vigorously overnight. Subsequently, the top (aqueous) layer was decanted, and the bottom (ionic liquid) layer was washed three times with an aqueous solution of LiNTf2 (0.02 mol cm−3) and ten times with deionised water. Remaining solvents were removed using a rotary evaporator (80 °C, 1 h) and then high vacuum (80 °C, overnight, 10−2 mbar).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, before being placed in the microwave reactor Anton Parr Monowave 300 for 1 min. Reaction temperatures were varied between 200 and 280 °C (temperature measurement error by the IR probe was ±10 °C). The product formed was a suspension of a fine brown/black powder in an ionic liquid, and was subsequently washed with methanol and centrifuged to separate the particles from the ionic liquid.
4 ratio, before being placed in the microwave reactor Anton Parr Monowave 300 for 1 min. Reaction temperatures were varied between 200 and 280 °C (temperature measurement error by the IR probe was ±10 °C). The product formed was a suspension of a fine brown/black powder in an ionic liquid, and was subsequently washed with methanol and centrifuged to separate the particles from the ionic liquid.
Characterisation
Scanning electron microscopy (SEM) studies were carried out using a JEOL 6500F Field Emission and a Quanta FEG 250 Scanning Electron Microscope. Energy dispersive X-ray (EDX) analysis was carried out using Oxford Instruments INCA systems.X-Ray diffraction (XRD) data were collected using a Siemens D5000 powder diffractometer with Cu Kα radiation (γ = 1.542 Å). Data were recorded from between 10° and 60° in steps of 0.0167.
Raman spectra were recorded using a PerkinElmer RamanStation 400F spectrometer, with a 785 nm focussed laser beam. All studied samples were placed in quartz cuvettes and analysed neat; ten scans of 5 s each were recorded.
Results and discussion
In the approach presented here, diphenyl diselenide was dissolved in a chloroindate(III) ionic liquid and exposed to microwave radiation to result directly in microcrystalline indium(III) selenide. The specific ratio of reagents (3 moles of Ph2Se2 to 4 moles of ionic liquid with a [InCl4]− anion to give an In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratio of 2
Se ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was chosen to achieve the desirable stoichiometry of In2Se3. The metal-containing ionic liquid acts as a non-volatile solvent and reactant at the same time in an ionothermal process.
3) was chosen to achieve the desirable stoichiometry of In2Se3. The metal-containing ionic liquid acts as a non-volatile solvent and reactant at the same time in an ionothermal process.
Under ambient conditions diphenyl diselenide was suspended in an ionic liquid, but upon heating between 50 and 60 °C, all ionic liquids readily dissolved diphenyl diselenide, Ph2Se2, to form homogenous, orange/yellow solutions (Fig. 1a). Upon further heating (240 to 280 °C), a brown to black suspension was formed, as shown in Fig. 1b.
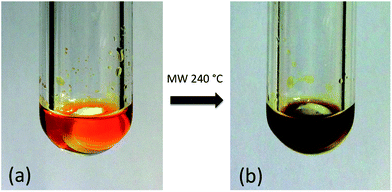 | ||
| Fig. 1 Solution of diphenyl diselenide in the chloroindate ionic liquid (a) and the reaction mixture after 1 min exposure to microwave radiation at 240 °C (b). | ||
Raman spectra of the solution of Ph2Se2 in the ionic liquid are compared to the spectra of the neat ionic liquid and pure Ph2Se2 (Fig. 2). The Raman spectrum of the neat ionic liquid displays vibrations at 224 and 114 cm−1, which are characteristic of the tetrahedral [InCl4]− anion.39 For the spectrum of Ph2Se2 dissolved in the ionic liquid (Fig. 2a), peaks are evident for both the ionic liquid and the selenium precursor, and it appears that the Ph2Se2 simply dissolves in the ionic liquid, without coordination to the indium centre. When the mixture is allowed to cool, the Ph2Se2 precipitates out of the ionic liquid and forms two phases again.
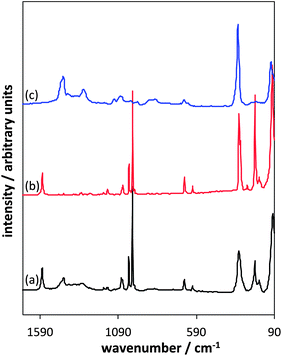 | ||
Fig. 2 Raman spectra of: the solution of Ph2Se2 in [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] (a), diphenyl diselenide (b) and neat [P6 17][InCl4] (a), diphenyl diselenide (b) and neat [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] (c). 17][InCl4] (c). | ||
For all solutions it was found that the reaction was completed after just 1 min in a microwave reactor. In various sets of experiments, the influence of the following factors:
• reaction temperature,
• ionic liquid cation,
• dilution with an ‘inert’ ionic liquid,
• speciation of the ionic liquid anionon the composition and morphology of indium(III) selenide microcrystals was studied.
The influence of temperature
In the first set of experiments, solutions of Ph2Se2 in four chloroindate(III) ionic liquids: [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4], [P6
10][InCl4], [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14][InCl4], [P6
14][InCl4], [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] and [C8mim][InCl4] were heated in a microwave reactor for 1 min. For each solution, five reaction temperatures were tested: 200, 210, 220, 240 and 280 °C. No reaction was observed under 220 °C, neither by visual assessment, nor by Raman spectroscopy. For reaction temperatures of 240 and 280 °C, the ionic liquid solutions were visibly different after the reaction: they changed from a yellow homogeneous solution to a dark brown/black suspension (Fig. 1). In addition, [P8
17][InCl4] and [C8mim][InCl4] were heated in a microwave reactor for 1 min. For each solution, five reaction temperatures were tested: 200, 210, 220, 240 and 280 °C. No reaction was observed under 220 °C, neither by visual assessment, nor by Raman spectroscopy. For reaction temperatures of 240 and 280 °C, the ionic liquid solutions were visibly different after the reaction: they changed from a yellow homogeneous solution to a dark brown/black suspension (Fig. 1). In addition, [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4] was used to prepare indium(III) selenide at the previously determined optimum temperature of 240 °C.
5][InCl4] was used to prepare indium(III) selenide at the previously determined optimum temperature of 240 °C.
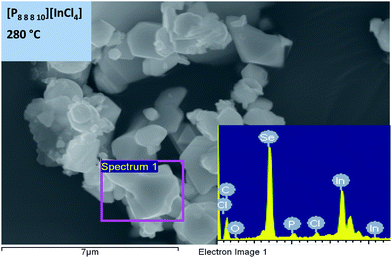
Morphologies of the microcrystals prepared at both temperatures, with different cations, were studied using SEM; furthermore, EDX was used to elucidate elemental composition and stoichiometry of the products formed (Table 1).
| No. | Ionic liquid | Morphology (by SEM) | Elemental composition (by EDX, wt%) | ||
|---|---|---|---|---|---|
| 240 °C | 280 °C | 240 °C | 280 °C | ||
| 1 | [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4] 10][InCl4] |
Uniform spherical product, 0.5–3 μm | Non-uniform 1–3 μm, 10–30 μm | 48.44% In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.56% Se 51.56% Se |
1% P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3% Cl 3.3% Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77.5% Se 77.5% Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.5% In 19.5% In |
| 2 | [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14][InCl4] 14][InCl4] |
Uniform product, 2–5 μm | Non-uniform, 10–30 μm, affected by laser, no clear image | Prod. 1: 38.7 In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59.12 Se 59.12 Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.75 O 4.75 O |
0.465% P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.145% Cl 2.145% Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82.6% Se 82.6% Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.25% In 14.25% In |
Prod. 2: 26.47 In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71.83% Se 71.83% Se |
|||||
| 3 | [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] 17][InCl4] |
1–3 μm, clear uniform product | 1–3 μm, clear uniform product | 48.26% In, 50.64% Se | 91.1% Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.9% In 8.9% In |
| 4 | [C8mim][InCl4] | 0.05–1 μm, clear uniform spherical product | 1–5 μm, unclear, non uniform product | Prod. 1: 100% Se | Prod. 1: 100% Se |
| Prod. 2: 69.7% Se, 30.3% In | Prod. 2: 4.57% Cl, 58.6% Se, 0.423% In, 0.36% N | ||||
| 5 | [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4] 5][InCl4] |
0.05–1 μm, clear uniform spherical product | — | Prod. 1: 50.1% In, 49.9% Se | — |
Table 1 shows that, at 280 °C, [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4], [P6
10][InCl4], [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14][InCl4] and [P6
14][InCl4] and [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] gave very similar results, yielding products of neither uniform nor well-defined size and morphology (Fig. 3). From the EDX analysis, for all samples prepared at 280 °C, an excess of selenium relative to indium was found, which suggests the formation of polyselenides. A small amount of phosphorus was also evident, indicating the incorporation of the phosphonium cation from the ionic liquid. The weight percentages of the elements may suggest the formation of anionic selenido-indates, such as the previously reported compounds [PR4][In(Se4)] or [PR4][In2(Se4)4(Se5)].40
17][InCl4] gave very similar results, yielding products of neither uniform nor well-defined size and morphology (Fig. 3). From the EDX analysis, for all samples prepared at 280 °C, an excess of selenium relative to indium was found, which suggests the formation of polyselenides. A small amount of phosphorus was also evident, indicating the incorporation of the phosphonium cation from the ionic liquid. The weight percentages of the elements may suggest the formation of anionic selenido-indates, such as the previously reported compounds [PR4][In(Se4)] or [PR4][In2(Se4)4(Se5)].40
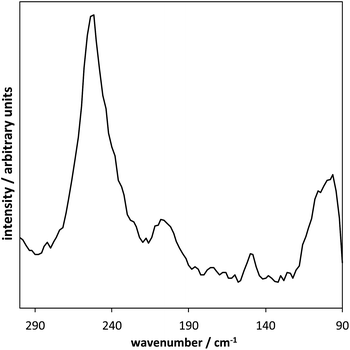
The main peak found in the Raman spectra for the products of reactions carried out at 280 °C was found at 254 cm−1, which is indicative of a polyselenide (vibration formed from α-monoclinic Se–Se) – see Fig. 4. A small peak, which corresponds to the presence of γ-In2Se3, was found at 150 cm−1.8 [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] used at 280 °C produced indium selenide without organic inclusions, but the composition determined by EDX corresponded to a polyselenide structure of the [In(Se)4]40 formula. Finally, regarding the [C8mim][InCl4] ionic liquid, elemental selenium was detected, and the inclusion of the ionic liquid into the product was indicated by the presence of nitrogen in the EDX analysis.
17][InCl4] used at 280 °C produced indium selenide without organic inclusions, but the composition determined by EDX corresponded to a polyselenide structure of the [In(Se)4]40 formula. Finally, regarding the [C8mim][InCl4] ionic liquid, elemental selenium was detected, and the inclusion of the ionic liquid into the product was indicated by the presence of nitrogen in the EDX analysis.
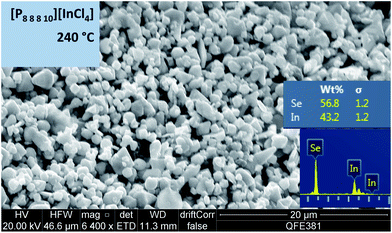
The reaction at 240 °C using [C8mim][InCl4] resulted in both elemental selenium and an indium selenide compound (with stoichiometry close to In2Se3) present in the final product. In contrast, the phosphonium ionic liquids tested at 240 °C gave products containing exclusively indium and selenium in various stoichiometries, in the absence of any ‘contamination’ by the ionic liquid cation. In particular, [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4], [P6
10][InCl4], [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] and [P8
17][InCl4] and [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4] yielded materials with the In
5][InCl4] yielded materials with the In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se weight ratio very close to the desired In2Se3, which is 51% In
Se weight ratio very close to the desired In2Se3, which is 51% In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49% Se, with a clear uniform microcrystalline morphology (Fig. 5). [P6
49% Se, with a clear uniform microcrystalline morphology (Fig. 5). [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14][InCl4] gave a mixture of indium selenides of varying stoichiometries. Building on these observations, 240 °C was selected as the optimum temperature for further studies.
14][InCl4] gave a mixture of indium selenides of varying stoichiometries. Building on these observations, 240 °C was selected as the optimum temperature for further studies.
The influence of ionic liquid cation
The study revealed that with varying temperatures, the cation had a marked influence on both composition and morphology of the product, which is in agreement with the literature.30–32 The [C8mim]+ cation yielded materials of poor morphology and composition, irrespective of the reaction temperature, while the phosphonium cations appeared to yield materials of uniform morphology. Phosphonium ionic liquids have numerous advantages over the imidazoloium based analogues, including a greater stability (thermal, chemical and electrochemical), a larger electrochemical window, and a lower cost. They also have a lower viscosity than ammonium analogues, with small amounts of dilutants having a profound effect on the viscosity of the ionic liquids under investigation.41The influence of dilution with a spectator ionic liquid
It was established that using a reaction temperature of 240 °C, with [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4] as a solvent for the dissolution of the selenium precursor, the optimum conditions to prepare indium(III) selenide are achieved. In attempt to further investigate the control of the morphology of the products, the influence of dilution was investigated.
10][InCl4] as a solvent for the dissolution of the selenium precursor, the optimum conditions to prepare indium(III) selenide are achieved. In attempt to further investigate the control of the morphology of the products, the influence of dilution was investigated.
The solution of Ph2Se2 in [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4] was diluted with [P8
10][InCl4] was diluted with [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][NTf2]. This ionic liquid was chosen to have a common cation with the chloroindate(III) precursor and an almost inert, weakly coordinating anion. The dilutions were prepared in two mass ratios: 1
10][NTf2]. This ionic liquid was chosen to have a common cation with the chloroindate(III) precursor and an almost inert, weakly coordinating anion. The dilutions were prepared in two mass ratios: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The SEM-EDX results showed that, in addition to reducing the product size from microcrystalline to nanosize, the stoichiometry was compromised and resulted in the production of a polyselenide, which is undesirable for the use in semiconductor applications. The reaction was also repeated using [P6
2. The SEM-EDX results showed that, in addition to reducing the product size from microcrystalline to nanosize, the stoichiometry was compromised and resulted in the production of a polyselenide, which is undesirable for the use in semiconductor applications. The reaction was also repeated using [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4], with the results giving no benefits to the current reaction, the morphology remained the same, with a detrimental effect on the stoichiometry.
17][InCl4], with the results giving no benefits to the current reaction, the morphology remained the same, with a detrimental effect on the stoichiometry.
The influence of ionic liquid anion
Another set of reactions was carried out to investigate the effect of the anion on the stoichiometry of the product as well as its size and morphology. Besides tetrachloroindate(III), two other chloroindate(III) anions are known:28 [InCl5]2− and [InCl6]3−. Ionic liquids based on these anions were formed using [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]Cl and appropriate stoichiometries of InCl3. Solutions of Ph2Se2 in the two chloroindate(III) ionic liquids, [P6
14]Cl and appropriate stoichiometries of InCl3. Solutions of Ph2Se2 in the two chloroindate(III) ionic liquids, [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]2[InCl5] and [P6
14]2[InCl5] and [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]3[InCl6], were heated in a microwave for 1 min. For each solution, six reaction temperatures were tested: 200, 210, 220, 240, 260 and 280 °C. In both cases, no reaction was detected between 200 and 260 °C, neither judging by the appearance of the solution nor by Raman spectroscopy. At 280 °C, the products were suspensions of dark brown/black powder in an ionic liquid. The precipitate obtained was analysed by SEM/EDX, Raman spectroscopy and PXRD.
14]3[InCl6], were heated in a microwave for 1 min. For each solution, six reaction temperatures were tested: 200, 210, 220, 240, 260 and 280 °C. In both cases, no reaction was detected between 200 and 260 °C, neither judging by the appearance of the solution nor by Raman spectroscopy. At 280 °C, the products were suspensions of dark brown/black powder in an ionic liquid. The precipitate obtained was analysed by SEM/EDX, Raman spectroscopy and PXRD.
The Raman spectra of both products prepared at 280 °C (see Fig. 6) indicated that indium(III) selenide was formed, with the distinct peak found at 150 cm−1, which is characteristic of γ-In2Se3.
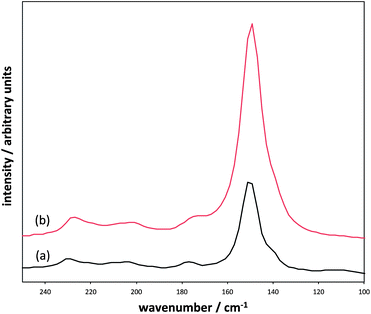 | ||
Fig. 6 Comparison of Raman spectra of the products formed when using [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]3[InCl6] + Ph2Se2 (a) and product formed when using [P6 14]3[InCl6] + Ph2Se2 (a) and product formed when using [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]2[InCl5] + Ph2Se2 (b). 14]2[InCl5] + Ph2Se2 (b). | ||
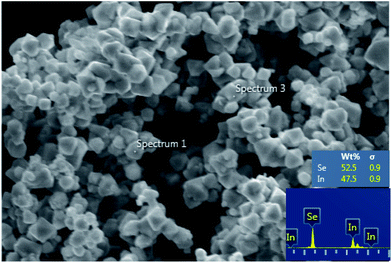
The SEM/EDX analysis show that for the reaction involving [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]2[InCl5], the product was microcrystalline indium(III) selenide, with a uniform size and morphology (Fig. 7). When using [P6
14]2[InCl5], the product was microcrystalline indium(III) selenide, with a uniform size and morphology (Fig. 7). When using [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]3[InCl6], the product was less well-defined and contained two materials of varying ratios of In
14]3[InCl6], the product was less well-defined and contained two materials of varying ratios of In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se, with the bulk of the material formed being In2Se3.
Se, with the bulk of the material formed being In2Se3.
These results indicate that aside from changing the phosphonium cation, also the anion can be varied in order to produce the desired stoichiometry of the indium selenide product. As shown above, many of the tested cations produced In2Se3 at 240 °C, but with [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14][InCl4] it was not possible. However, using the [P6
14][InCl4] it was not possible. However, using the [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]2[InCl5] and [P6
14]2[InCl5] and [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14]3[InCl6] ionic liquids, and applying a higher temperature of 280 °C, In2Se3 was successfully synthesised.
14]3[InCl6] ionic liquids, and applying a higher temperature of 280 °C, In2Se3 was successfully synthesised.
Phase identification
Powder XRD patterns for the products synthesised at 280 °C showed no resemblance to any indium selenide polymorph, or to any known polyselenides. N.B., there was only one published example of a polyselenide containing indium, selenium and phosphorus, of the stoichiometry In4P6Se18, and its XRD pattern differed greatly from the products formed at 280 °C. In contrast, samples prepared at 240 °C in ionic liquids based on the following cations: [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4] (Fig. 8b), [P6
10][InCl4] (Fig. 8b), [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] (Fig. 8c), [P8
17][InCl4] (Fig. 8c), [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4] (Fig. 8d), gave XRD patterns in close agreement with that of the In2Se3 standard (Fig. 8a).
5][InCl4] (Fig. 8d), gave XRD patterns in close agreement with that of the In2Se3 standard (Fig. 8a).
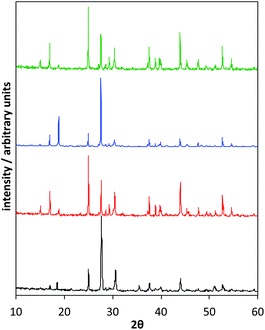 | ||
Fig. 8 Comparison of XRD spectra; In2Se3 standard (a), products when using [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4] + Ph2Se2 (b), [P6 10][InCl4] + Ph2Se2 (b), [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] + Ph2Se2 (c), [P8 17][InCl4] + Ph2Se2 (c), [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4] + Ph2Se2 (d). 5][InCl4] + Ph2Se2 (d). | ||
The distribution and intensities of the peaks indicate that the γ form of In2Se3 has been synthesised, with the main peaks found at 27° (006) and 58° (0012).42 Moreover, the experimental XRD patterns are identical with the simulated pattern for γ-In2Se3.
The Raman spectrum obtained for each of these products was in very good agreement with the XRD study, indicating the presence of γ-In2Se3. The Raman spectra of the products prepared at 240 °C, using [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4], [P6
10][InCl4], [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] and [P8
17][InCl4] and [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4], featured the characteristic peak of γ-In2Se3 at 150 cm−1. This peak also matches the dominant signal found for the In2Se3 standard (Fig. 9). However, the purchased standard appears to be of the κ-In2Se3 form, which has a similar molecular structure to γ-In2Se3, but with a more equal distribution between the peaks at 150 cm−1 and 250 cm−1.8
5][InCl4], featured the characteristic peak of γ-In2Se3 at 150 cm−1. This peak also matches the dominant signal found for the In2Se3 standard (Fig. 9). However, the purchased standard appears to be of the κ-In2Se3 form, which has a similar molecular structure to γ-In2Se3, but with a more equal distribution between the peaks at 150 cm−1 and 250 cm−1.8
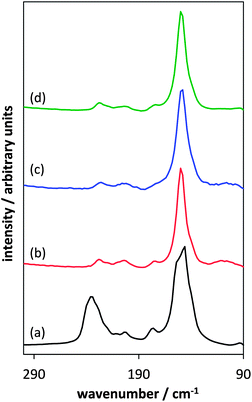 | ||
Fig. 9 Comparison of Raman spectra of the In2Se3 standard (a), and the products prepared using [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10][InCl4] + Ph2Se2 (b), [P6 10][InCl4] + Ph2Se2 (b), [P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17][InCl4] + Ph2Se2(c) and [P8 17][InCl4] + Ph2Se2(c) and [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5][InCl4] + Ph2Se2 (d). 5][InCl4] + Ph2Se2 (d). | ||
Conclusions
A new route towards indium(III) selenide has been successfully developed. It involves inexpensive phosphonium ionic liquids, used in a safe and green ionothermal procedure. The reaction temperature has been lowered by at least 60 °C compared to the literature methods, and the reaction time has been reduced from several hours to 1 min, thanks to the employment of a microwave reactor. The need for organic solvents has been eliminated. To our knowledge, this is the first example of using either ionic liquids and the ionothermal approach, or microwave-based synthesis, to prepare indium(III) selenide.From the combination of SEM, EDX, Raman and XRD studies, it is conclusive that γ-In2Se3 has been produced in each case. The novel microwave-assisted synthesis of indium(III) selenide has allowed for a more simple, less time consuming and elegant way to make this semiconductor material.
Acknowledgements
The authors would like to thank Michael Moser, Jeff Dyck and the team at CYTEC for all their support and abundance of phosphonium ionic liquid supplies and Dr John D. Holbrey for access to the microwave reactor. Dr Gabriela Adamova is acknowledged for samples of [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5]Cl and [P8
5]Cl and [P8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10]Cl. P.N. thanks the EPSRC for a RCUK fellowship.
10]Cl. P.N. thanks the EPSRC for a RCUK fellowship.
Notes and references
- H. M. Pathan, S. S. Kulkarni, R. S. Mane and C. D. Lokhande, Mater. Chem. Phys., 2005, 93, 16 CrossRef CAS PubMed.
- S. Yang, H. Wang, W. Fu and D. Kelley, J. Photochem. Photobiol., A, 2007, 192, 159 CrossRef CAS PubMed.
- I. H. Mutlu, M. Z. Zarbaliyev and F. Aslan, J. Sol-Gel Sci. Technol., 2007, 43, 223 CrossRef CAS PubMed.
- H. Bouzouita, N. Bouguila, S. Duchemin, S. Fiechter and A. Dhouib, Renewable Energy, 2002, 25, 131 CrossRef CAS.
- A. Chaiken, K. Nauka, G. A. Gibson, H. Lee, C. C. Yang, J. Wu, J. W. Ager, K. M. Yu and W. Walukiewicz, Appl. Phys. Lett., 2003, 94, 2390 CAS.
- J. Ye, S. Soeda, Y. Nakamura and O. Nittono, Jpn. J. Appl. Phys., 2008, 37, 4264 CrossRef.
- J. Jasinski, W. Swider, J. Washburn, Z. Liliental-Weber, A. Chaiken, K. Nauka, G. A. Gibson and C. C. Yang, J. Appl. Phys., 2002, 81, 1 Search PubMed.
- C. deGroot and J. Moodera, J. Appl. Phys., 2001, 89, 4336 CrossRef CAS PubMed.
- J. J. Ning, G. J. Xiao, N. R. Xiao, L. Wang, B. B. Liu and B. Zou, J. Cryst. Growth, 2011, 336, 1 CrossRef CAS PubMed.
- P. Vaqueiro, Inorg. Chem., 2008, 47, 20 CrossRef CAS PubMed.
- A. K. Cheetham, G. Ferey and T. Loiseau, Angew. Chem., Int. Ed., 1999, 38, 3284 CrossRef.
- K. Tsamartzi, J. H. Song, T. Bakas, A. J. Freeman, P. N. Trikalitis and M. G. Kanatzidis, Inorg. Chem., 2008, 47, 11920 CrossRef PubMed.
- C. Wang, X. H. Bu, N. F. Zhang and P. Y. Feng, Angew. Chem., Int. Ed., 2002, 41, 1959 CrossRef CAS.
- S. M. Yang and D. F. Kelley, J. Phys. Chem. B, 2005, 109, 12701 CrossRef CAS PubMed.
- R. Sreekumar, Phys. Status Solidi B, 2013, 250 Search PubMed.
- J. L. Lu, Y. Xie, F. Xu and L. Zhu, J. Mater. Chem., 2002, 12, 2755 RSC.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS.
- K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351 CrossRef CAS.
- J. S. Wilkes, J. A. Levinsky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263 CrossRef CAS.
- P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. Van Hecke, L. Van Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110, 20978 CrossRef CAS PubMed.
- E. Ahmed, J. Breternitz, M. F. Groh and M. Ruck, CrystEngComm, 2012, 14, 4874 RSC.
- E. Ahmed, A. Isaeva, A. Fiedler, M. Haft and M. Ruck, Chem. – Eur. J., 2011, 17, 6847 CrossRef CAS PubMed.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471 RSC.
- E. Ahmed and M. Ruck, Coord. Chem. Rev., 2011, 255, 2892 CrossRef CAS PubMed.
- Z. Lin, D. S. Wragg, J. E. Warren and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 10334 CrossRef CAS PubMed.
- D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050 CrossRef CAS PubMed.
- J. D. Holbrey, J. Estager and M. Swadźba-Kwaśny, Chem. Soc. Rev., 2014, 43 10.1039/C3CS60310E.
- D. C. Apperley, C. Hardacre, P. Licence, R. W. Murphy, N. V. Plechkova, K. R. Seddon, G. Srinivasan, M. Swadźba-Kwaśny and I. J. Villar-Garcia, Dalton Trans., 2010, 39, 8679 RSC.
- A. Taubert and Z. Li, Dalton Trans., 2007, 723 RSC.
- (a) A. Taubert, P. Steiner and A. Mantion, J. Phys. Chem. B, 2005, 109, 15542 CrossRef CAS PubMed; (b) A. Taubert, C. Palivan, O. Casse, F. Gozzo and B. Schmitt, J. Phys. Chem. C, 2007, 111, 4077 CrossRef CAS.
- J. Estager, P. Nockemann, K. R. Seddon, G. Srinivasan and M. Swadźba-Kwaśny, ChemSusChem, 2011, 5, 117 CrossRef PubMed.
- C. J. Serpell, J. Cookson, A. L. Thompson, C. M. Brown and P. D. Beer, Dalton Trans., 2013, 42, 1385 RSC.
- K. Biswas, Q. Zhang, I. Chung, J.-H. Song, J. Androulakis, A. J. Freeman and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 14760 CrossRef CAS PubMed.
- K. Biswas, I. Chung, J.-H. Song, C. D. Malliakas, A. J. Freeman and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 5657 CrossRef CAS PubMed.
- E. Ahmed and M. Ruck, Coord. Chem. Rev., 2011, 255, 2892 CrossRef CAS PubMed.
- Z. Ma, J. Yu and S. Dai, Adv. Mater., 2010, 22, 261 CrossRef CAS PubMed.
- S. Mallakpour and Z. Rafiee, Prog. Polym. Sci., 2011, 36, 1754 CrossRef CAS PubMed.
- G. Adamová, R. L. Gardas, L. P. N. Rebelo, A. J. Robertson and K. R. Seddon, Dalton Trans., 2011, 40, 12750 RSC.
- (a) J. Yang, P. Tian, L. He and W. Xu, Fluid Phase Equilib., 2003, 204, 295 CrossRef CAS; (b) J. Yang, P. Tian, W. Xu, B. Xu and S. Liu, Thermochim. Acta, 2004, 412, 1 CrossRef CAS PubMed.
- S. S. Dhinga and M. G. Kanatzidis, Inorg. Chem., 1993, 32, 1357 Search PubMed.
- K. J. Fraser and D. R. MacFarlane, Aust. J. Chem., 2009, 62, 209 CrossRef.
- A. M. Rasmussen, S. T. Teklemichael, E. Mafi, Y. Gu and M. D. McCluskey, Appl. Phys. Lett., 2013, 102, 062105 CrossRef PubMed.
- S. J. Ewing, A. V. Powell and P. Vaquiero, J. Solid State Chem., 2011, 184, 1800 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |