 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxygen reduction and methanol oxidation behaviour of SiC based Pt nanocatalysts for proton exchange membrane fuel cells†
Rajnish
Dhiman
*a,
Serban N.
Stamatin
a,
Shuang M.
Andersen
a,
Per
Morgen
b and
Eivind M.
Skou
a
aDepartment of Chemical Engineering, Biotechnology and Environmental Technology, University of Southern Denmark (SDU), Niels Bohrs Alle 1, DK-5230 Odense M, Denmark
bDepartment of Physics, Chemistry and Pharmacy, University of Southern Denmark (SDU), Campusvej 55, DK-5230 Odense M, Denmark. E-mail: rdhiman@sdu.dk; Fax: +45-6615 8760; Tel: +45-6550 23603
First published on 16th October 2013
Abstract
Research with proton exchange membrane fuel cells has demonstrated their potential as important providers of clean energy. The commercialization of this type of fuel cell needs a breakthrough in the electrocatalyst technology to reduce the relatively large amount of noble metal platinum used with the present carbon based substrates. We have recently examined suitably sized silicon carbide (SiC) particles as catalyst supports for fuel cells based on the stable chemical and mechanical properties of this material. In the present study, we have continued our work with studies of the oxygen reduction and methanol oxidation reactions of SiC supported catalysts and measured them against commercially available carbon based catalysts. The deconvolution of the hydrogen desorption signals in CV cycles shows a higher contribution of Pt (110) and Pt (111) peaks compared to Pt (100) for SiC based supports than for carbon based commercial catalysts, when HClO4 is used as an electrolyte. The Pt (110) and Pt (111) facets are shown to have higher electrochemical activities than Pt (100) facets. To the best of our knowledge, methanol oxidation studies and the comparison of peak deconvolutions of the H desorption region in CV cyclic studies are reported here for the first time for SiC based catalysts. The reaction kinetics for the oxygen reduction and for methanol oxidation with Pt/SiC are observed to be similar to the carbon based catalysts. The SiC based catalyst shows a higher specific surface activity than BASF (Pt/C) for methanol oxidation and oxygen reduction while the mass activity values are comparable.
Introduction
Fuel cells are becoming the current focus of energy research due to their high efficiency and for being environmentally friendly. The state of the art fuel cells use platinum as a catalyst which contributes around 30–45% to the total cost,1 thus making this technology very expensive. A catalyst in the form of Pt nanoparticles is uniformly dispersed over the support to enhance the catalytic surface area and reduce the amount of catalyst needed. Several different alloys of Pt have been investigated with different metals such as Pd, Ni, Co, Cr, Sn, Ru1,2etc. as a possible choice for enhancing the catalytic activity and reducing the cost of the fuel cells. Apart from catalysts, a catalyst support is also an important avenue of research in order to commercialize the fuel cells. Carbon black is the standard catalyst support used in PEMFCs to obtain an optimized catalytic activity at a reasonable cost.3,4 Generally, a good catalyst support material should have chemical inertness, high mechanical strength, high thermal stability, good electrical conductivity and high surface area. Other forms of carbon, which were proven to be more corrosion resistant,5 are graphitized carbon black, carbon nanofibers, carbon nanotubes5–7 and other graphene related materials.8 These materials can be produced with high conductivity and specific surface areas. However, the carbon black supports are susceptible to electrochemical oxidation especially at the fuel cell cathode.9,10 The carbon corrosion leads to the migration and agglomeration of catalyst particles.9 This process results in the decrease of electrochemical surface area (ESA) and it affects the performance of the PEMFCs. Thus some alternative support materials are highly desired to enhance the stability of the catalysts.11Different ceramic materials have been investigated for applications as catalyst supports in fuel cells. Titanium based materials such as TiB2,12 TiO2 13 and TiN nanoparticles14 have been investigated and reported to show good electrochemical activity. Nikiforov et al.15 studied the electrochemical activity of IrO2 supported on SiC–Si composites and they observed an increased activity of the catalyst. Lv et al.4 studied the electrocatalytic activity of Pt/SiC and Pt/SiC/C and they found good electrocatalytic performance for Pt/SiC/C. In our recent work,16 we also observed a slightly higher ESA for Pt/SiC catalysts in comparison with commercially used carbon black based catalysts (BASF), where Pt nanoparticles are deposited over the SiC nanocrystals by the polyol method, using K2PtCl4 as the metal precursor unlike in the work by Lv et al.
Reduction of Pt-loadings will help to commercialize the fuel cell technology.17 Pt-loadings have successfully been decreased from 0.2–0.4 mgPt cm−2 to 0.05 mgPt cm−2 for the anode electrode18 because of the large activity of Pt towards the H2-oxidation reactions. The reaction kinetics of the oxygen reduction reaction (ORR) involve multi-electron reactions along with several elementary steps,1 thus requiring higher overpotentials. This complex ORR kinetics along with poor activity of Pt for ORR pose hurdles for reduction of the Pt-loading (0.4 mgPt cm−2) on cathodes.18 Thus it makes for the ORR to be among the most studied electrocatalytic reactions. It is important to study the ORR of Pt based electrocatalysts on different catalyst supports. Also, the methanol oxidation reaction (MOR) is a slow process in comparison with the hydrogen oxidation reactions (at least three to four orders of magnitude slower).19 MOR involves the transfer of six electrons to the electrode for the complete oxidation to carbon dioxide. Several intermediates are formed during the methanol oxidation such as CO, formic acid, formaldehyde20etc. Some species such as CO irreversibly adsorb on the surface of the electrocatalyst thus decreasing the surface area available for adsorbing CH3OH and severely poison the Pt for the rest of the reaction. This also leads to a decrease in the rate of oxidation. Thus, new electrocatalysts are required to inhibit the poisoning and to increase the rate of oxidation.
SiC has certain unique properties such as good chemical and mechanical properties, thermal stability, and acid corrosion resistance,16 which perfectly fits with the requirements for a good support material. In the present work, we have studied the oxygen reduction reaction and methanol oxidation behavior of SiC based supports.
Experimental methods
Materials and sample preparation
The Pt/SiC electrocatalysts are prepared by depositing platinum on SiC substrates by using the polyol method. The method of Pt deposition on SiC materials has been reported previously.16 The SiC materials in different forms and shapes are used as substrates. The details of the three different SiC materials are given in Table 1. Apart from these Pt/SiC materials, a commercially available Pt/C (20 wt% Pt) catalyst (BASF, LOT # H0020626) has also been tested for comparison with electrochemical behavior of Pt/SiC (20 wt% Pt). The catalyst, Pt/C catalyst (BASF, LOT # H0020626), is mentioned as BASF in the whole manuscript.| Sample name (type) | Particle size | Specific surface area (SSA)/m2 g−1 | Synthesis route |
|---|---|---|---|
| a The particles have been assumed as spheres, thus the specific surface area has been calculated by dividing the surface area by the mass of particles. | |||
| SiC–NS (nanoporous SiC powder) | 25–35 nm | Calculateda as 54–75 m2 g−1 | Synthesized by the reaction of carbon black with SiO vapors16,21 at a temperature 1450 °C |
| SiC–SPR (SiC nanocrystals) | 50–150 nm | Calculateda as 18.7 m2 g−1 using 100 nm as the diameter | Synthesized by the solid phase reaction of silicon with carbon black at a temperature of 1525 °C 16 |
| SiC–SMS (microporous SiC powder) | 1–10 μm | BET surface area measured to be in the range of 11.82–21.2 m2 g 21 | Synthesized by the reaction of carbon with SiO vapors, where the carbon is obtained by the carburization of wood21 |
The aqueous acid electrolyte solutions were prepared from perchloric acid (70% Fluka TraceSelect®, for trace analysis) and ultrapure Milli-Q® (Millipore, MA USA) water with a resistivity of not less than 18 MΩ cm. ORR kinetics were investigated using a rotating disk electrode (RDE). SiC supported platinum nano-catalyst samples were deposited on the RDE surface. The catalysts of around 2–3 mg were mixed in a sufficient amount of Milli-Q® water and sonicated for around 30 min. The amount of water was decided upon according to the targeted Pt loading on the RDE surface. The final catalyst loading was around 120 μgPt cm−2 for SiC based electrodes, while 30 μgPt cm−2 was used for BASF. In order to achieve full coverage of the RDE for ORR, we optimized a loading of 120 μgPt cm−2 for Pt/SiC due to the larger density and smaller BET surface area of SiC in comparison with the active carbon supports. A drop of 50 μL suspension was put over the mirror polished glassy carbon (GC) and left to dry under an IR lamp.
Electrochemical methods and setup
The electrochemical CV measurements were performed using a Zahner IM6eX electrochemical workstation (Zahner-Electric GmBH & Co. KG, Germany) controlled by a Thales 1.48. The RDE setup used is from Pine Research Instruments and it consists of an AFMSRXE modulated speed rotator and a tip (E5 RDE), and also a 5.0 mm diameter (geometric surface area of 0.1964 cm2) mirror polished glassy carbon electrode surface. The RDE along with the catalyst dispersed onto the GC surface was used as the working electrode in a two compartment, three electrode set up. A reversible hydrogen electrode (RHE) from Gaskatel GmbH, Germany, was used as the reference electrode (RE), while a platinum wire of 0.3 mm thickness wound to 40 windings constituting approximately 3 mm diameter, sealed in a glass tube with a ceramic frit, was used as the counter electrode (CE). All the measurements were performed at a temperature of 25 °C, which was kept constant with the help of a thermostat. All potentials listed are with respect to RHE.ORR studies were conducted in a two compartment, three electrode setup filled with 200 ml of 0.5 M HClO4. The background was collected at 0.05 V s−1 from 0.05 to 1.3 V in an Ar saturated electrolyte. ORR was recorded in an O2 saturated electrolyte at 0.05 V s−1 for 400, 900, 1600 and 2500 rpm. Both gases were purged at 20 ml min−1 for 30 min. The working electrode was cleaned from 0.01 to 1.6 V until a stable signal was obtained. In order to measure the kinetic current the measurements recorded in Ar were subtracted from the ones in O2.
MOR was studied in a total volume of 200 ml containing 0.25 M HClO4 and 0.25 M methanol. Ar was purged for 30 min at 20 ml min−1. All the measurements were recorded after subsequent cleaning of the working electrode by cycling until a stable CV is obtained. MOR was recorded in the potential range of 0 to 1.4 V, at a scan rate of 10 mV s−1. The investigated catalyst was drop coated on a 0.12 cm2 Au electrode which was connected by a gold wire as the working electrode. All the data were processed by using OriginPro 8.5® (Originlab, USA).
Calculations
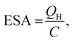 | (1) |
 | (2) |
 | (3) |
 | (4) |
In eqn (4), n is the number of electrons transferred, F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.53 Cmol−1), A is the area of the electrode used, co and Do are respectively the concentration and diffusion coefficient of oxygen in the bulk electrolyte, and v is the kinematic viscosity of the liquid phase.
485.53 Cmol−1), A is the area of the electrode used, co and Do are respectively the concentration and diffusion coefficient of oxygen in the bulk electrolyte, and v is the kinematic viscosity of the liquid phase.
 | (5) |
Results and discussion
Catalyst characterization
The XRD patterns of the different Pt/SiC catalysts shown in Fig. 1 display the peaks corresponding to SiC and Pt. The peaks at 2θ = 35.6°, 41.3°, 60.0°, 71.7° and 75.4° correspond to diffraction from SiC (111), (200), (220), (311) and (222) planes respectively, while the peaks at 39.7°, 46.2° and 67.5° appear due to the diffraction from (111), (200), and (220) planes of Pt. The average Pt crystallite size was estimated by the Scherrer formula using the full width at half maximum (FWHM) of the peak at 39.7°. The calculation of the Pt crystallite size using the Scherrer formula gives values of 3.7 nm, 5 nm and 5.8 nm for SiC–NS (nano-porous), SiC–SPR (nanocrystals) and SiC–SMS (micro-porous) respectively. | ||
| Fig. 1 XRD patterns of SiC nanocrystals and the Pt/SiC electrocatalysts. (a) nanocrystals synthesized by solid phase reaction, (b) nanoporous SiC synthesized by reaction with silicon monoxide and (c) microporous SiC powder. | ||
Fig. 2 and 3 show respectively the SEM and TEM images of Pt/SiC catalysts synthesized upon different SiC based supports. Pt nanoparticles appear light grey to white in contrast to the darker SiC particles in SEM images while Pt nanoparticles appear darker in TEM images compared to SiC. The SiC substrates have been masked by the Pt particles. The size of the Pt nanoparticles in the samples SiC–SMS and SiC–SPR is in the range of 3–8 nm while for SiC–NS the size range is 3–5 nm. The calculation of the average size of samples (based upon TEM images) gives the average values of 3.9 ± 2.3, 5.5 ± 2.6 and 7.0 ± 2.0 nm for SiC–NS, SiC–SMS and SiC–SPR respectively. The histograms of particle size distribution are given in Fig. S2, ESI.† The comparison of the respective SEM and TEM images (especially Pt/SiC–SMS with Pt/SiC–NS and Pt/SiC–SPR) indicates that the Pt is distributed evenly over the supports irrespective of the differences in the size of support particles.
 | ||
| Fig. 2 SEM images of the Pt/SiC electrocatalysts at different magnifications. (a) and (b) show SEM images of Pt nanoparticles deposited over SiC–SMS (Pt/SiC–SMS), (c) and (d) show images of Pt catalyst on SiC–NS (Pt/SiC–NS) and SiC–SPR (Pt/SiC–SPR) respectively. | ||
 | ||
| Fig. 3 TEM images of the electrocatalysts Pt/SiC at different magnifications. (a), (b) and (c) are TEM images of Pt/SiC–SMS at different magnifications and different regions, (d) and (e) are images of Pt/SiC–NS and Pt/SiC–SPR respectively. | ||
The detailed characterization of Pt/SiC–NS and Pt/SiC–SPR was reported previously.16 The XPS analysis of Pt/SiC–SMS is given in the ESI.†
Electrochemical characterization
The sequence of electrochemical activity in HClO4 shows the following trend Pt (110) > Pt (111) > Pt (100).30Fig. 4 shows the peak deconvolution of the CVs of different samples in the H desorption region. Deconvolution of the desorption region curve is done by fitting the peaks as Gaussians using OriginPro 8.5®. Table 2 shows the ratio of the Pt (110 and 111) peaks to the Pt (100) peak. The ratios of the peaks have been calculated from the H desorption curves for the different samples (shown in Fig. 4). It is clear from Fig. 4 and Table 2 that SiC based samples have higher contribution from the Pt (110) and Pt (111) facets in comparison with the platinized carbon based commercial sample BASF in the HClO4 electrolyte.
 | ||
| Fig. 4 Peak deconvolution of the cyclic voltammograms of different samples in the H desorption region. The Pt loading on electrodes is 30 μg cm−2 for BASF and 120 μg cm−2 for SiC based catalyst samples. | ||
| Sample name | Ratio of Pt(110 and 111) to Pt(100) peaks |
|---|---|
| Pt/SiC–NS | 3.54 |
| Pt/SiC–SMS | 2.20 |
| Pt/SiC–SPR | 2.02 |
| BASF | 1.37 |
The ECSA values for different SiC based catalysts in comparison with commercial BASF are shown in Table 3 (also reported in our earlier work16). The ECSA values do not reflect the Pt particle sizes. It is well known that on Pt/C (Vulcan), some Pt particles are not accessible for electrochemical reactions. We believe that the above mentioned inconsistency is caused by a similar effect.
| Sample name | Pt loading (%) | ECSAa (m2 g−1) | SA (mA cm−2) | MAb (A mg−1) |
|---|---|---|---|---|
| a ECSA has been calculated by dividing ESA by the Pt electrode loading. b MA has been calculated by dividing the kinetic current by Pt electrode loading. | ||||
| BASF | 20 | 62.2 | 0.29 | 0.18 |
| Pt/SiC–SMS | 20 | 38.6 | 0.25 | 0.12 |
| Pt/SiC–NS | 20 | 49.7 | 0.36 | 0.18 |
| Pt/SiC–SPR | 20 | 39.2 | 0.28 | 0.14 |
Kinetic currents have been calculated using eqn (3) by extrapolating the reciprocal current (shown in Fig. 5(e)) to the zero on ω−1/2 axes (infinite rotation); the intercept at the y-axis is the kinetic current while the slope of the extrapolated line is used to find the number of electrons transferred per oxygen molecule on the catalyst. All the SiC based samples show similar or higher SA than BASF while the mass activity (MA, see Table 3) of Pt/SiC–NS is the same as the BASF and less for the other two samples. Similar values of MA and SA were reported for 20 wtPt% Pt/C by Gasteiger et al.18 The number of electrons transferred at 0.9 V (vs. RHE) as calculated for all tested samples are 3.7, 3.7, 3.8 and 3.9 for BASF, Pt/SiC–SMS, Pt/SiC–NS and Pt/SiC–SPR respectively. Thus it confirms the 4 e− pathway for the ORR for all the catalysts. Fig. 5(f) and S2† show a significant shift in the ORR onset for Pt/SiC compared to the commercial Pt/C (BASF). The Tafel plots for all the tested samples are shown in Fig. S4 (ESI).† As a whole, the slopes of the SiC based catalysts are similar to the BASF, indicating similar reaction kinetics for ORR.
 | ||
| Fig. 5 (a–d) The anodic sweeps of ORR voltammograms of different catalysts in 0.5 M HClO4 in the 0.4–1.2 V vs. RHE at a scan rate of 50 mV s−1, (a) Pt/SiC–SMS, (b) Pt/SiC–NS (c) Pt/SiC–SPR and (d) BASF at different rotating speeds of RDE. The Koutecky–Levich plots used to calculate the kinetic currents are given in (e) while (f) shows the linear sweep voltammograms for all electrocatalysts in O2 saturated 0.5 M HClO4; 0.05 V s−1, 1600 rpm. | ||
 | ||
| Fig. 6 Cyclic voltammograms of different Pt/SiC and BASF catalysts in 0.5 M CH3OH at 10 mV s−1. | ||
Conclusions
In the present study, we have investigated the electrochemical behavior of SiC based catalysts and compared it with the commercially available Vulcan based catalyst BASF. We have studied the oxygen reduction reaction and methanol oxidation along with the deconvolution of H desorption peaks of the CV curves. The comparison of the deconvoluted H desorption peaks of Pt/SiC to Pt/C is being reported for the first time. We observed higher contribution of the Pt(110 and 111) signal than Pt(100) in the case of different SiC based catalysts in comparison with BASF. The electrochemical activity is higher for the Pt(110) and Pt(111) than for Pt(100) in HClO4. The surface activity of the SiC based catalysts is found to be higher for one sample while comparable for others and the mass activity is found to be comparable for one while smaller for others. Overall, the reaction kinetics for all the tested samples including BASF are found to be similar. The methanol oxidation behaviour of SiC based catalysts has also been reported for the first time and the results obtained are similar to the ORR results. The surface specific activity of SiC based catalysts is higher than the BASF while the mass specific activity of one SiC based sample is comparable to BASF and slightly smaller for others. Thus, the overall conclusion of the electrochemical studies shows SiC based catalysts as better than or equally competent to BASF.Acknowledgements
The authors are grateful to the Danish Ministry for Research and Innovation for providing a grant to R. Dhiman, 2104-05-0073 through its program for Sustainable Energy and The Environment. They are further grateful to the Danish PSO project CATBOOSTER 2011-1-10669. The authors are thankful to T. Warner, P. B. Hansen, D. Kyrping, and T. Sørensen at SDU for advice and technical support.Notes and references
- A. Rabis, P. Rodriguez and T. J. Schmidt, ACS Catal., 2012, 2, 864–890 CrossRef CAS.
- N. M. Markovic, T. J. Schmidt, V. Stamenkovic and P. N. Ross, Fuel Cells, 2001, 1, 105–116 CrossRef CAS.
- T. R. Ralph and M. P. Hogarth, Platinum Met. Rev., 2002, 46, 3–14 CAS.
- H. Lv, S. Mu, N. Cheng and M. Pan, Appl. Catal., B, 2010, 100, 190–196 CrossRef CAS PubMed.
- J. Y. Cheon, C. Ahn, D. J. You, C. Pak, S. H. Hur, J. Kim and S. H. Joo, J. Mater. Chem. A, 2013, 1, 1270 CAS.
- M. J. Larsen and E. M. Skou, J. Power Sources, 2012, 202, 35–46 CrossRef CAS PubMed.
- S. M. Andersen, M. Borghei, P. Lund, Y.-R. Elina, A. Pasanen, E. Kauppinen, V. Ruiz, P. Kauranen and E. M. Skou, Solid State Ionics, 2013, 231, 94–101 CrossRef CAS PubMed.
- E. Antolini, Appl. Catal., B, 2009, 88, 1–24 CrossRef CAS PubMed.
- N. Cheng, S. Mu, M. Pan and P. P. Edwards, Electrochem. Commun., 2009, 11, 1610–1614 CrossRef CAS PubMed.
- L. M. Roen, C. H. Paik and T. D. Jarvi, Electrochem. Solid-State Lett., 2004, 7, A19–A22 CrossRef CAS PubMed.
- D. A. Stevens and J. R. Dahn, Carbon, 2005, 43, 179–188 CrossRef CAS PubMed.
- S. Yin, S. Mu, H. Lv, N. Cheng, M. Pan and Z. Fu, Appl. Catal., B, 2010, 93, 233–240 CrossRef CAS PubMed.
- S. Huang, P. Ganesan, S. Park and B. N. Popov, J. Am. Chem. Soc., 2009, 131, 13898–13899 CrossRef CAS PubMed.
- B. Avasarala, T. Murray, W. Li and P. Haldar, J. Mater. Chem., 2009, 19, 1803–1805 RSC.
- A. V. Nikiforov, T. A. L. Garcia, I. M. Petrushina, E. Christensen and N. J. Bjerrum, Int. J. Hydrogen Energy, 2011, 36, 5797–5805 CrossRef CAS PubMed.
- R. Dhiman, E. Johnson, E. M. Skou, P. Morgen and S. M. Andersen, J. Mater. Chem. A, 2013, 1, 6030 CAS.
- I. E. L. Stephens, A. S. Bondarenko, U. Grønbjerg, J. Rossmeisl and I. Chorkendorff, Energy Environ. Sci., 2012, 5, 6744 CAS.
- H. A. Gasteiger, S. S. Kocha, B. Sompalli and F. T. Wagner, Appl. Catal., B, 2005, 56, 9–35 CrossRef CAS PubMed.
- A. S. Aricò, S. Srinivasan and V. Antonucci, Fuel Cells, 2001, 1, 133–161 CrossRef.
- R. Chetty, W. Xia, S. Kundu, M. Bron, T. Reinecke, W. Schuhmann and M. Muhler, Langmuir, 2009, 25, 3853–3860 CrossRef CAS PubMed.
- R. Dhiman, V. Petrunin, K. Rana and P. Morgen, Ceram. Int., 2011, 37, 3281–3289 CrossRef CAS PubMed.
- M. Søgaard, M. Odgaard and E. M. Skou, Solid State Ionics, 2001, 145, 31–35 CrossRef.
- T. J. Schmidt, H. A. Gasteiger, G. D. Stab, P. M. Urban, D. M. Kolb and R. J. Behm, J. Electrochem. Soc., 1998, 145, 2354–2358 CrossRef CAS PubMed.
- U. A. Paulus, T. J. Schmidt, H. A. Gasteiger and R. J. Behm, J. Electroanal. Chem., 2001, 495, 134–145 CrossRef CAS.
- J. Munk, P. A. Christensen, A. Hamnett and E. Skou, J. Electroanal. Chem., 1996, 401, 215–222 CrossRef.
- C. Paoletti, A. Cemmi, L. Giorgi, R. Giorgi, L. Pilloni, E. Serra and M. Pasquali, J. Power Sources, 2008, 183, 84–91 CrossRef CAS PubMed.
- A. B. Kashyout, A. B. A. A. Nassr, L. Giorgi and T. Maiyalagan, Int. J. Electrochem. Sci., 2011, 6, 379–393 CAS.
- D. M. Gattia, M. V. Antisari, L. Giorgi, R. Marazzi, E. Piscopiello, A. Montone, S. Bellitto, S. Licoccia and E. Traversa, J. Power Sources, 2009, 194, 243–251 CrossRef PubMed.
- T. Frelink, W. Visscher and J. A. R. van Veen, Electrochim. Acta, 1995, 40, 545–549 CrossRef CAS.
- J. C. Meier, C. Galeano, I. Katsounaros, A. A. Topalov, A. Kostka, F. Schu and K. J. J. Mayrhofer, ACS Catal., 2012, 2, 832–843 CrossRef CAS.
- C. Zhou, H. Wang, F. Peng, J. Liang, H. Yu and J. Yang, Langmuir, 2009, 25, 7711–7717 CrossRef CAS PubMed.
- L. Giorgi, T. D. Makris, R. Giorgi, N. Lisi and E. Salernitano, Sens. Actuators, B, 2007, 126, 144–152 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Basic theory of oxygen reduction reaction, detailed information about the instruments used for characterization, detailed XPS analysis of Pt/SiC-SMS and additional images of Tafel plots and cyclic voltammograms are given in the ESI along with some additional CVs of the catalysts. See DOI: 10.1039/c3ta12744c |
| This journal is © The Royal Society of Chemistry 2013 |
