 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unique norbornene polymer based "in-field" sensor for As(III)†
Sourav
Bhattacharya‡
,
Santu
Sarkar‡
and
Raja
Shunmugam
*
Indian Institute of Science Education and Research, Kolkata, Polymer Research Centre, Department of Chemical Sciences, Mohanpur, West Bengal, India. E-mail: sraja@iiserkol.ac.in
First published on 28th May 2013
Abstract
We report the synthesis of norbornene derived rhodamine (Nor-Rh) and thiol (Nor-Th) monomers and their polymerizations. The response of Nor-Rh against As(III) in the presence of potassium iodate and hydrochloric acid is monitored through UV and fluorescence spectroscopy. The observed colorimetric as well as fluorometric changes are attributed to the redox reaction involved in the unique design. Cyclic voltammogram measurements confirm the oxidation of As(III) to As(V) during the sensing event. The response of the sensing event is instantaneous. This novel design is able to sense ppb levels of As(III) very selectively in an aqueous environment. As(III) is trapped when it is passed through a column containing Nor-Th polymers. As(III) free water is tested using a calibration curve from the Nor-Rh response.
Arsenic enters drinking water mostly in the form of inorganic trivalent (arsenite, As(III)) and pentavalent (arsenate, As(V)) oxidation states from natural deposits in the earth or from agricultural and industrial practices.1 The combination of high toxicity and widespread occurrence has created a pressing need for effective monitoring and measurement of arsenic in a variety of environmental samples such as soil and groundwater.2 To protect consumers from the effects of long term chronic exposure to arsenic compounds, WHO and EPA have prescribed that the Maximum Contaminant Level of As(III) in the water used for consumption should not be more than 0.010 parts per million (10 parts per billion).3a,b Conventional and frequently used methods (hydride generation atomic absorption spectrometry (HG-AAS), stripping voltammetry, neutron activation analysis and X-ray fluorescence) have induced high cost as well as some operational difficulties.4–9 Though Gutzeit methods have been extensively used for field tests, the major concern here is the generation of highly toxic arsine gas.10 Spectrophotometric methods based on the formation of molybdoarsenate are not so effective in the application to environmental samples.11
Although the modern fluorescence detection techniques12 provide a simple and viable approach for the detection of biologically relevant analytes such as Zn(II), Hg(II), Cd(II) and Pb(II), until now there is no effective solution for As(III). The chemical properties of As compounds make their binding to sensor chromophores very difficult.3a A very recent report demonstrates a coumarin-6 based sensing system for the first time but it is applicable only in organic solvents.3b
Until now, there is no simple and viable technique for the field determination of arsenic in aqueous environments.13 We have tried an innovative approach by the combination of colorimetry and fluorimetry in an aqueous environment to produce a norbornene derived rhodamine monomer (Nor-Rh) that responds very selectively and sensitively to As(III) in the presence of potassium iodate and hydrochloric acid. The procedure is very simple and easy to follow. It involves very basic redox chemistry where As(III) is oxidized to As(V) by reducing iodate to iodine. Due to this, the solution changes its color from pink to brown colorimetrically and red-orange to green under the UV light. The newly designed system merely uses the very basic redox reaction in the sensing process. The response time is instantaneous even at ppb level of As(III) and the redox reaction is very specific to As(III). To the best of our knowledge this is the first report that effectively senses As(III) in aqueous environments.
 | ||
| Scheme 1 Synthesis of Nor-Rh and its polymer PNor-Rh. | ||
Sensing As(III) by a simple and efficient method is one of the biggest challenges for researchers.14 There is no viable technology available today for the efficient removal of As(III) compared to As(V).2 Due to this, As(III) is converted to As(V) by a pre-oxidation method.2 Some well-known effective oxidizers are chlorine, ferric chloride, and potassium permanganate which can easily oxidize As(III) to As(V).2 It is also known that potassium iodate oxidizes As(III) to As(V) by liberating the iodine in the presence of acid.15 This prompted us to design our unique molecule to sense As(III) effectively. In this article, we report a new norbornene based rhodamine monomer (Nor-Rh) which responds to As(III) visibly and fluorometrically in the presence of potassium iodate and hydrochloric acid.
We believe that while sensing, the system oxidizes As(III) to As(V) by liberating iodine. The unique colorimetric as well as fluorimetric responses are clearly attributed to the liberated iodine's coordination with the Nor-Rh.
First of all, rhodamine B was converted to rhodamine amine.16 Then it was coupled with norbornene functionalized acid using DCC as a coupling agent (Scheme 1). Purification of the product was conducted through precipitation in methanol followed by ether. 1H NMR spectroscopy confirmed the formation of pure product where the signals were clearly indicative of the norbornene double bond and the characteristic aromatic signals were on account of the rhodamine motif (Fig. S1†). It was very well supported by the 13C NMR spectroscopy. CHN analysis and mass spectroscopy further supported the formation of the monomer in the pure form (Fig. S2†). After confirming the formation of the pure monomer (Nor-Rh), we focused on its polymerization to obtain PNor-Rh. Unfortunately, after the addition of Grubbs' catalyst to Nor-Rh in DCM, the color of the reaction mixture immediately changed to pink and became fluorescent as well. Obviously this was due to the ring opened form of rhodamine. It is known in literature that presence of Fe(III) could open the spirolactam ring of rhodamine to make it fluorescent.17 Ru(III) present in the Grubbs' catalyst, (being in the same group of Fe in the periodic table) might be doing a similar role. Hence we slightly changed our synthetic route to make PNor-Rh by post polymer modification (Fig. S6–S8†).18 It is also known from the literature that the ring closed form of rhodamine is colorless and non-luminescent (Fig. S11†). But in the open form, which is normally induced by either acid or some metal ions,19 rhodamine is pink in color and highly luminescent as well. After confirming the formation Nor-Rh and PNor-Rh, the colorless nature of both Nor-Rh and PNor-Rh clearly suggested the closed form of the spirolactam ring in the rhodamine motif.
Sensing properties of the Nor-Rh monomer towards As(III) were explored in presence of KIO3 and HCl. A stock solution of Nor-Rh was prepared in methanol–water mixtures whereas As(III), and KIO3 were prepared in water. First we monitored the response of sensor (1) with As(III) by UV-visible spectroscopy. As soon as a drop of HCl was added to the mixture of Nor-Rh and KIO3 an immediate pink color was developed. UV spectroscopy confirmed the characteristic absorbance band of rhodamine at 560 nm due to the spirolactam ring opening. Then the As(III) solution was gradually added dropwise to the sensing solution 1. A new absorbance band was observed at 288 nm and 356 nm with increasing intensity whereas the absorbance at 560 nm gradually decreased with increased concentration of the As(III) solution (Fig. 1a). A characteristic isosbestic point was observed at 520 nm and the color of the solution became brown as shown in Fig. 1b. The observed color change was very unique and interesting since the very strong pink color of rhodamine completely vanished due to the As(III) addition.
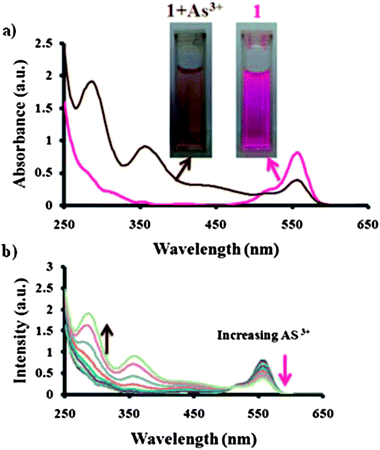 | ||
| Fig. 1 (a) UV-Vis spectra along with the colorimetric change of solution 1 before and after the addition of As(III). (b) UV-Visible spectra of solution 1 while titrating with As(III). | ||
Next, the emission properties of sensing solution 1 were explored with the increasing concentration of As(III). Fig. 2a shows the change in the emission of solution 1 with the gradual addition of As(III). Solution 1 initially showed a characteristic rhodamine emission at 640 nm when it was excited at 560 nm. With each addition of As(III), there was a shift of 5 nm from the initial emission wavelength towards the blue region, as shown in Fig. 2b. Finally, a blue shift of 40 nm from its initial emission of solution 1, (from 640 nm to 600 nm) was observed with gradual addition of As(III). The change of emission could be clearly visible from red-orange to green (before and after the addition of As(III)) under the UV lamp, as shown in Fig. 2a. The observed unusual colorimetric and fluorometric change of Nor-Rh in solution 1 while sensing As(III), prompted us to explore the importance of the design. First we wanted to confirm the redox reaction between KIO3 and As(III) in the presence of Nor-Rh.
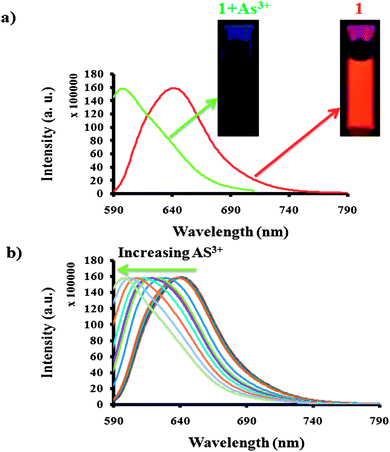 | ||
| Fig. 2 (a) Emission spectra of solution 1 before and after the addition of As(III) and it's colour change under a hand-held UV-lamp; (b) blue shift in emission spectra of solution 1 while titrating with As(III) (λex = 560 nm). | ||
To prove this, we performed two important control experiments. First, we measured the pH of solution 1 upon gradual addition of As(III). Interestingly, it was observed that the pH of the solution 1 with addition of As(III) increased from 1.34 to 4.23 (Fig. S13†). When the pH of the solution reached the value above 4, the color of the solution turned into brown. We attributed the observed increase in pH which was due to the consumption of acid for the redox reaction between As(III) and KIO3 to get As(V) and I2 (Fig. S16†). Before the redox reaction, the presence of acid opened the ring in Nor-Rh, hence there was absorbance at 560 nm. Next, the redox reaction was strongly supported by cyclic voltammetry (CV) experiments (Fig. 3). From the CV experiments it was observed that a two electron oxidation was involved which was responsible for the two humps, one at 0.425 V and another at 0.804 V. It was interesting to observe from the cyclic voltammogram that the redox couple was reversible as well (Fig. S14†). Sensing experiments were carried out with different metal solutions to ensure the selectivity of the sensing procedure towards As(III). Various metal salts like Co(II), Ni(II), Hg(II), Cd(II), Zn(II), Na(I), Ca(II), Mn(II), Mg(II), As(III), Cu(II) and Pb(II) were taken at the same concentration. Since the redox reaction was unique for As(III) and KIO3, it was obvious that among all metals only As(III) responded to this particular sensing technique. From the UV spectra of all the metal ions tested, only As(III) showed the characteristic peaks at 288 nm and 356 nm. This was clearly reflected in the bar diagram where As(III) showed an intense peak whereas the other metals did not (Fig. 4a). A similar bar diagram from the emission spectra of all metal ions was recorded. Only in the case of As(III) a shift towards the blue region was observed (Fig. S17†). A detection limit of 200 nM of As(III) was observed using our unique system 1 (Fig. 4b).
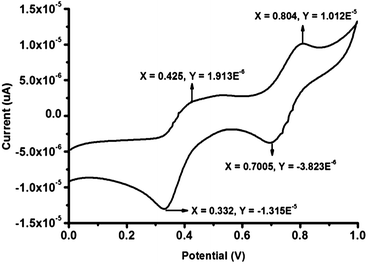 | ||
| Fig. 3 Cyclic voltammogram of As(III) and 1. | ||
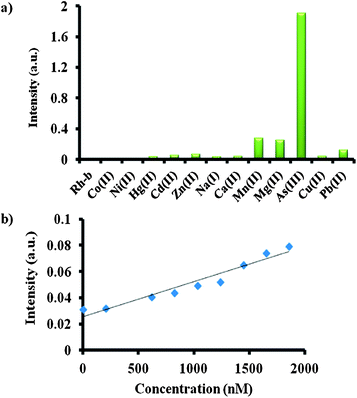 | ||
| Fig. 4 (a) Bar diagram representation of the selective responses for As(III) among all the metal ions by UV-visible spectroscopy; (b) detection limit experiment of As(III). | ||
After confirming the redox reaction between KIO3 and As(III), questions were asked to understand the observed brown (colorimetric) as well as green (fluorometric) response of Nor-Rh with As(III). In the literature, normal rhodamine based sensors always showed either colorless to pink (colorimetric) or no emission to red-orange emission (fluorometric) as a response to the addition of metal cations. But in our case, it was very unusual to observe a unique response from Nor-Rh upon the addition of As(III) to the sensing solution 1. The absorbance at 560 nm due to rhodamine decreased with increasing the concentration of As(III). Also, new absorbances at 450 nm, 356 nm and 288 nm were observed. In the fluorescence spectrum, there was a 40 nm blue shift from the characteristic rhodamine emission (640 nm to 600 nm) upon the addition of As(III) to the sensing solution 1. We thought about the role of the As(III) molecule's interaction with Nor-Rh, but As(III) already was involved in the redox reaction with KIO3 to generate As(V) (Fig. 5a). So the direct interaction of As(III) with Nor-Rh was not possible. This prompted us to study the possibility of the liberated I2 molecule's role in the observed response. To understand that, we carried out the same sensing procedure (KIO3, HCl, As(III)) with just rhodamine amine alone but the expected response was not observed in UV spectroscopy. We then repeated the procedure with norbornene acid (Nor-A).
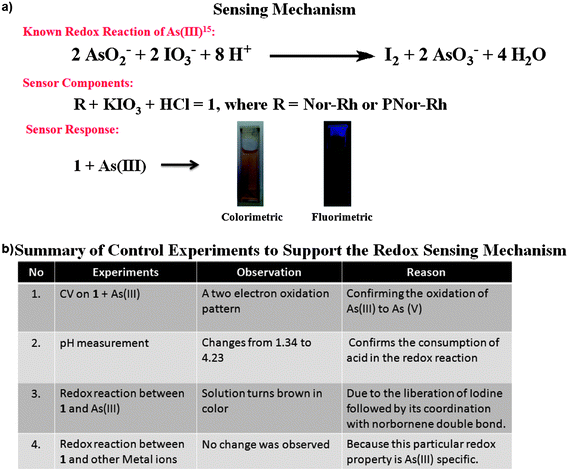 | ||
| Fig. 5 (a) Schematic representation of sensor components and sensing response; (b) summary of control experiments carried out to support the redox sensing mechanism. | ||
Again, the expected brown color was not observed. Instead, a very weak yellowish color was observed due to the molecular iodine. Only the norbornene attached rhodamine (Nor-Rh) showed the characteristic brown color as well as the green emission under the sensing conditions. We believed that this was due to the adduct formation between the liberated I2 and norbornene double bond. We hypothesized a possible halogen bonding formation (Fig. 8b) between the norbornene–I2 adduct and the rhodamine carbonyl oxygen.22 To prove this hypothesis, 1H NMR and IR spectroscopy experiments of Nor-Rh and 1 + As(III) were carried out. From 1H NMR analysis, a new signal was observed at 3.6 ppm in 1 + As(III) solution (Fig. 6). This was attributed to the signal from the norbornene–I2 adduct. Also the rhodamine aromatic protons were shifted downfield due to the spirolactam ring opening of the rhodamine motif. FT-IR spectroscopy showed a 12 cm−1 shift of the amide bond of the rhodamine motif due to the iodine interaction (Fig. 7). All the results are summarized in Fig. 8c.
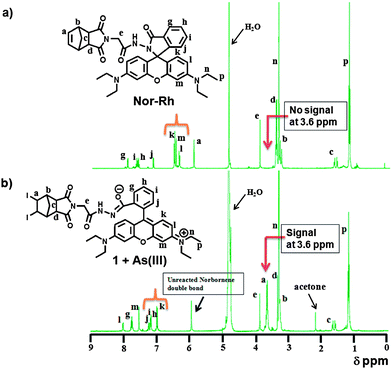 | ||
| Fig. 6 1H NMR spectrum of (a) Nor-Rh; (b) 1 + As(III). | ||
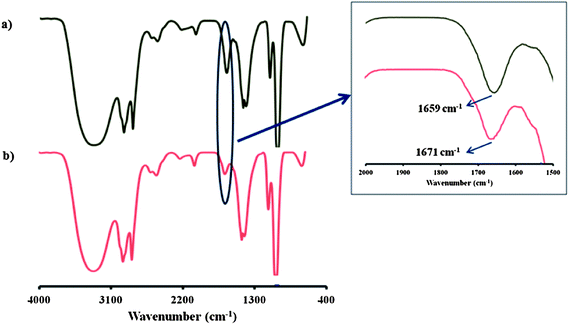 | ||
| Fig. 7 FT-IR spectrum of (a) Nor-Rh; (b) 1 + As(III). | ||
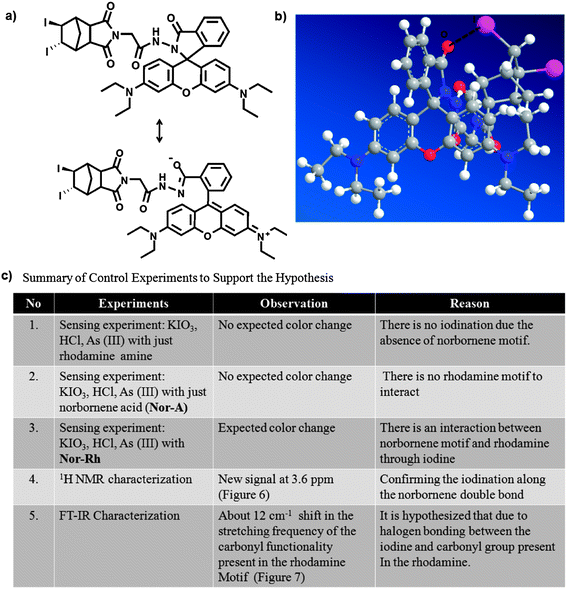 | ||
| Fig. 8 (a) Proposed chemical reaction of 1 + As(III); (b) proposed halogen bonding between iodine and the carbonyl functionality of the rhodamine motif; (c) summary of the control experiments that support our hypothesis. | ||
Next, to demonstrate the suitability of a practical device using this sensing system, paper strips were prepared by coating with a solution of PNor-Rh, KIO3 and HCl and were then dried well. A detailed synthetic procedure for making PNor-Rh is reported in the ESI.† The paper strips, coated with the sensor component, were dipped into different concentrations of arsenic solution at the ppb level. It was observed that with increasing arsenic concentration, the intensity of the brown color increased as shown in Fig. 9c. Interestingly, the change of color was instantaneous and prominent to the naked eye (ESI Video†). A calibration curve was made from the UV-vis spectroscopy response of 1 (characteristic absorbance at 288) with different concentrations of arsenic(III). Using this calibration curve the unknown concentration of the As(III) sample was identified. The concentration of the unknown As(III) samples were simultaneously checked by using an ICP-MS instrument (Fig. 9a) as well. It was very exciting to note that both the results were in excellent agreement (Fig. 9b). The results also showed the advantage of our design over other analytical techniques including ICP-MS since no extra effort was needed for the sample preparation.
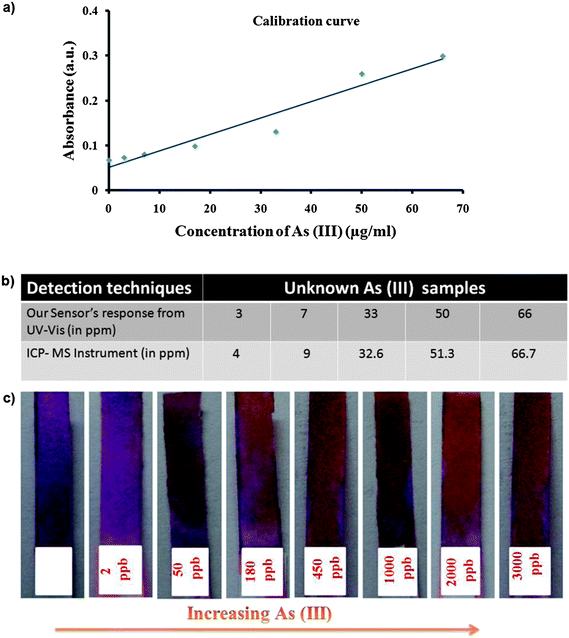 | ||
| Fig. 9 Comparisons of the efficiency of our sensing method to standard analytical methods; (a) a calibration curve is made from the UV-vis absorbance response of the sensing system with known concentrations of As(III); (b) a table summarizes the concentration of unknown As(III) samples obtained from our calibration curve as well as ICP-MS instrumentation; (c) our paper strip coated with 1 shows the response based on the concentration of As(III). | ||
Since As(III) has a specific affinity towards thiol functionality, a thiol based norbornene monomer (Nor-Th) and its polymer (PNor-Th) were synthesized to remove As(III) from the arsenic contaminated water (Scheme 2). A detailed synthetic procedure and complete characterization have been discussed in the ESI (Fig. S3–S5†).20 After preparing the pure Nor-Th, the corresponding polymer PNor-Th was prepared by Grubbs' 2nd generation catalyst via ROMP (Fig. S9 and S10†).21 Then the thiol group present in Nor-Th as well as PNor-Th was deprotected by hydrazine acetate before using them for arsenic removal. In a short column, PNor-Th was packed with alumina. 1 mL of a known concentration of As(III) containing water (3223 ppb) was passed through it. The eluent was collected from the column and the presence of arsenic was measured using ICP-MS instrumentation as shown in Fig. 10b. It was very interesting to note the As(III) content in the solution decreased from 3223 ppb to 1.2 ppb. This procedure was repeated over 5 cycles and up until the 5th cycle the column's efficiency in trapping As(III) remained almost same. For a comparison, we did another exciting experiment where we passed As(III) dissolved in water through a column packed with monomer (Nor-Th) and the polymer (PNor-Th), separately (Fig. S15†). As expected, the ability of the polymer in removing As(III) was 10 times greater than that of its corresponding monomer due to the availability of more thiol groups in the polymer to bind arsenic.
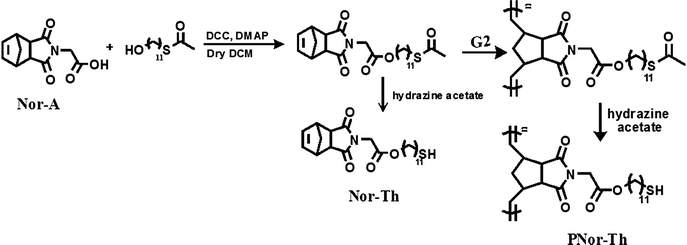 | ||
| Scheme 2 Synthesis of norbornene attached thiol monomer (Nor-Th) and polymer (PNor-Th). | ||
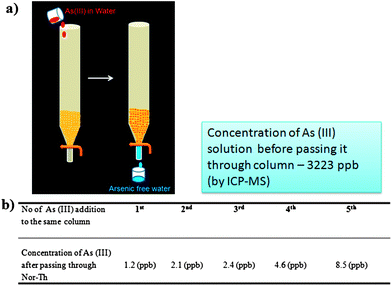 | ||
| Fig. 10 (a) A cartoon representation of As(III) removal; (b) a table summarizes the ICP-MS values after passing the As(III) solution through the Nor-Th containing column. | ||
In conclusion, a norbornene based rhodamine monomer (Nor-Rh) was synthesized and thoroughly characterized. This monomer and its polymer can selectively sense As(III) in aqueous environments in presence of KIO3 and HCl by colorimetry as well as fluorimetry. Polymer coated paper strips makes the compound more useful in the field of sensing. As arsenic(III) has a specific affinity towards thiol based ligands, a norbornene attached thiol monomer (Nor-Th) and its polymer (PNor-Th) have been used for removal applications.
Acknowledgements
SB and SS thank CSIR, New Delhi for the fellowship. RS thanks DST, New Delhi for the Ramanujan Fellowship and the Fast-Track Project. RS thanks IISER-Kolkata for the infrastructure. Dr Ravikant Vadlamani is acknowledged for his help in measuring the ICP-MS data.Notes and references
- J. C. Saha, A. K. Dikshit, M. Bandyopadhyay and K. C. Saha, Crit. Rev. Environ. Sci. Technol., 1999, 29, 281 CrossRef CAS.
- http://www.doe.gov/bridge .
- (a) R. K. Kalluri, T. Arbneshi, S. A. Khan, A. Neely, P. Candice, B. Varisli, M. Washington, S. McAfee, B. Robinson, S. Banerjee, A. K. Singh, D. Senapati and P. C. Ray, Angew. Chem., Int. Ed., 2009, 48, 1 CrossRef; (b) V. C. Ezeh and T. C. Harrop, Inorg. Chem., 2012, 51, 1213 CrossRef CAS.
- (a) M. Aarro, H. Lopez, M. C. Lopez and M. Sanchez, J. Anal. Toxicol., 1992, 116, 169 Search PubMed; (b) G. Samanta, D. Chakraborti and J. Fresenius, Fresenius' J. Anal. Chem., 1997, 357, 827 CrossRef CAS.
- N. Ybanez, M. L. Cervera and R. Montoro, Anal. Chim. Acta, 1992, 258, 61 CrossRef CAS.
- G. Samanta, A. Chaterjee, D. Das, P. P. Chowdhary, C. R. Chanda and D. Chakraborti, Environ. Technol., 1995, 16, 223 CrossRef CAS.
- D. Das, A. Chatterjee, G. Samanta and D. Chakraborti, Chem. Environ. Res., 1992, 1, 279 CAS.
- M. Shull and J. D. Winefordner, Anal. Chem., 1984, 56, 2617 CrossRef.
- Y. Madrid, D. Chakraborti and C. Camara, Microchim. Acta, 1995, 120, 63 CrossRef CAS.
- D. Melamed, Anal. Chim. Acta, 2005, 532, 1 CrossRef CAS.
- (a) T. P. Jahn and G. P. Bienert, in Advances in Experimental Medicine and Biology, Landes Bioscience, Austin, TX, 2010 Search PubMed; (b) W. R. Cullen and K. J. Reimer, Chem. Rev., 1989, 89, 713 CrossRef CAS.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210 RSC.
- A. Pillai, G. Sunita and V. K. Gupta, Anal. Chim. Acta, 2000, 408, 111 CrossRef CAS.
- (a) D. J. Thomas, Angew. Chem., 2009, 121, 1210 ( Angew. Chem., Int. Ed. , 2009 , 48 , 1188 ) CrossRef; (b) W. R. Cullen and K. J. Reimer, Chem. Rev., 1989, 89, 713 CrossRef CAS.
- B. Narayana, T. Cherian, M. Mathew and C. Pasha, Indian J. Chem. Technol., 2006, 13, 36 CAS.
- J. Du, J. Fan, X. Peng, P. Sun, J. Wang, H. Li and S. Sun, Org. Lett., 2010, 12, 476 CrossRef CAS.
- S. Wang, X. Meng and M. Zhu, Tetrahedron Lett., 2011, 52, 2840 CrossRef CAS.
- S. Sarkar, A. Mondal, A. K. Tiwari and R. Shunmugam, Chem. Commun., 2012, 48, 4223 RSC.
- S. Kang, S. Kim, Y.-K. Yang, S. Bae and J. Tae, Tetrahedron Lett., 2009, 50, 2010 CrossRef CAS.
- J. Yang, S. Rawat, T. L. Stemmler and B. P. Rosen, Biochemistry, 2010, 49, 3658 CrossRef CAS.
- (a) R. H. Grubbs Handbook of Metathesis, Wiley-VCH, New York, 2003, vol. 3 Search PubMed; (b) S. R. Mane, V. N. Rao and R. Shunmugam, ACS Macro Lett., 2012, 1, 482 CrossRef CAS; (c) S. Bhattacharya, V. N. Rao, S. Sarkar and R. Shunmugam, Nanoscale, 2012, 4, 6962 RSC; (d) A. E. Madkour, A. H. R. Koch, K. Lienkamp and G. N. Tew, Macromolecules, 2010, 43, 4557 CrossRef CAS.
- (a) W. S. Zou, J. Han and W. J. Jin, J. Phys. Chem. A, 2009, 113, 10125 CrossRef CAS; (b) M. Erdélyi, Chem. Soc. Rev., 2012, 41, 3547 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ta11587a |
| ‡ Both authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2013 |
