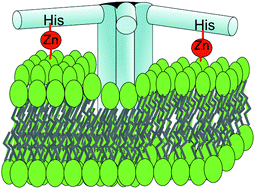
The mechanism of membrane permeabilization by dermcidin (DCD-1L), an antimicrobial peptide present in human sweat, was investigated at a mercury-supported monolayer of dioleoylphosphatidylcholine (DOPC) or dioleoylphosphatidylserine (DOPS) and at a mercury-supported tethered bilayer lipid membrane (tBLM) consisting of a thiolipid (DPTL) with a DOPC or DOPS monolayer self-assembled on top of it. In an unbuffered solution of pH 5.4, DCD-1L is almost neutral and permeabilizes a DPTL/DOPS tBLM at transmembrane potentials, ϕtrans, which are physiological. In a pH 7 buffer solution DCD-1L bears two negative charges and has no effect on a DPTL/DOPC tBLM, whereas it permeabilizes a DPTL/DOPS tBLM only outside the physiological ϕtrans range; however, the presence of zinc ion induces DCD-1L to permeabilize the DPTL/DOPS tBLM at physiological ϕtrans values. The effect of zinc ions suggests a DCD-1L conformation with its positive N-terminus embedded in the lipid bilayer and the negative C terminus floating on the membrane surface. This conformation can be stabilized by a zinc ion bridge between the His38 residue of the C terminus and the carboxyl group of DOPS. Chronocoulometric potential jumps from ϕtrans ≅ +160 mV to sufficiently negative values yield charge transients exhibiting a sigmoidal shape preceded by a relatively long “foot”. This behavior is indicative of ion-channel formation characterized by disruption of DCD-1L clusters adsorbed on top of the lipid bilayer, incorporation of the resulting monomers and their aggregation into hydrophilic pores by a mechanism of nucleation and growth.