 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reciprocal effects of the chirality and the surface functionalization on the drug delivery permissibility of carbon nanotubes
Amir Alipour
Skandani
and
Marwan
Al-Haik
*
Department of Engineering Science and Mechanics, Virginia Tech, Blacksburg, VA 24061, USA. E-mail: alhaik@vt.edu; Fax: +1 540 231 4574; Tel: +1 540 231 5442
First published on 24th October 2013
Abstract
The drug delivery admissibility of nanomaterials such as carbon nanotubes and their uncertain interactions with live tissues and organs have sparked ongoing research efforts. To boost the selective diffusivity of single walled carbon nanotubes (SWCNTs), surface functionalization was adopted in several experimental attempts. Numerous studies had identified polyethylene glycol (PEG) as a bio-compatible surfactant to carbon nanotubes. In this study, a large scale, atomistic molecular dynamic simulation was utilized to disclose the cellular exposure and uptake mechanisms of PEG-functionalized single walled carbon nanotubes (f-SWCNTs) into a lipid bilayer cell membrane. Results showed that with PEGs attached to a SWCNT, the penetration depth and speed can be controlled. Also, the simulations revealed that the adhesion energy between the nanotube and the lipid membrane is affected considerably, in the presence of PEGs, by the chirality of the SWCNTs.
Introduction
The foreseen application of carbon nanotubes (CNTs) as therapeutic transporters and diagnostic agents triggered a growing volume of research, both experimentally and computationally, in an attempt to tailor and better comprehend the phenomenological aspects of CNT–membrane interactions. The envisioned drug delivery capability of CNTs in general and single walled CNTs (SWCNTs) in particular can be attributed partially to their capability in transfecting through different lipid cell membranes in the human body.1 In our earlier study, it was shown that such penetration can be affected by different microstructural features of SWCNTs such as the length to diameter aspect ratio and the nanotube chirality and length.2 Carbon nanotubes, like all other foreign particles, once jabbed into the body should first be able to elude the immune system. The reticuloendothelial system (RES) is a monocular phagocytic system which can filter the alien objects (pathogens) from the blood stream by identifying blood serum components (opsonins) attached to them. Thereafter, tens of lysosomal enzymes and oxidative chemicals attack the opsonized particle for degradation.3 The immunogenicity and nonspecific uptake of CNTs hamper their drug delivery undertaking which most of the times can be resolved by surface functionalization.4 Single walled CNTs are potential vehicles that can be easily convened with proper chemical functionalities to carry biomolecules such as proteins, DNA and RNAi.1b,c,5 Based on clinical studies, different functionalizing agents could enhance the attachment of biomolecules to SWCNTs and also could reduce the nanotube cytotoxicity via better dispersion in water.5 An antibiofouling surface for increased circulation uptake and decreased non-specific interaction is needed for the application of therapeutic nanoparticles. Polyethylene glycol (PEG), a widely used polyether compound with applications spanning from industrial manufacturing to medicine and as a water soluble polymer, was utilized in several experimental investigations to functionalize different nanomaterials to promote both higher dispersibility and lower cytotoxicity.3a,6 However, due to unsophisticated characterization techniques for tracking down the particles in vivo, most of the knowledge earned so far is limited to in vitro clinical observations and ensuing symptoms in animals. This makes it inconclusive to determine the phenomenological aspects of functionalization and the functionalized particle interactions in the body. It is crucial to understand the mechanisms of cell internalization, trafficking and excretion of surface-modified SWCNTs to qualify them as safe and applicable drugs carriers.The non-specific physical penetration of the cell membrane, endocytosis or a combination of both is hypothesized to be the main mechanism for the CNT uptake phenomenon.2,7 The non-conclusive observations obtained by experimental studies – mainly due to the variations in the characterization techniques and errors imposed by diagnostic tools – necessitate the need for realistic simulation techniques such as molecular dynamics (MD). Molecular dynamics have been extensively exploited to mimic the SWCNT–cell membrane interaction within relatively short durations and they revealed several interesting features about the CNT internalization through the cell membranes.2,8 Few published studies have delineated the functionalization effects on the transfection of CNTs into cell membranes utilizing atomistic scale simulation techniques.8a,b,9 In this study, we report the results of large scale atomistic MD simulations on the effect of two interfacial properties of SWCNTs on their cell internalization. In particular, the effects of the SWCNT chirality and their surface functionalization with covalently bound PEG chains are investigated. Three different CNTs with distinct chiralities are chosen with identical total electrical charges and lengths with and without PEG chains attached to them.
PEGylated SWCNTs
The formulation and precise control of the targeting ligand density on the surfaces of therapeutic nanoparticles are vital due to their additional effect on the ‘stealth’ surface of nanoparticles. On the other hand, the effectiveness of the obtained bundles highly depends on the binding mechanisms of these ligands to the surfaces of the nanoparticles. It was reported that higher blood circulation times were achieved when PEG chains were covalently attached to the CNTs rather than being surface-adsorbed.5,10Fig. 1 illustrates the two commonly adopted chemical bindings between the PEG chains and SWCNTs. Experimentally, both bonding types can be achieved either by application of pristine materials after strong acid treatment or by 1, 3 dipolar cycloaddition reaction.3b However, the common protocol of functionalization is the former one where typical CNT surface oxidation is facilitated by an oxidizing agent such as piranha solution (a mixture of sulfuric acid and hydrogen peroxide) or by nitric acid.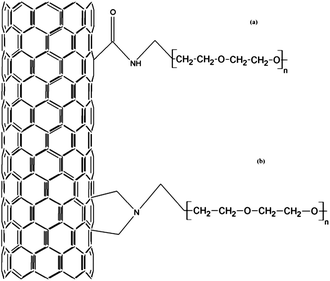 | ||
| Fig. 1 Common PEG–SWCNT intermediary chemical bonding protocols, (a) through carboxylic acid groups and (b) through 1, 3 dipolar cycloaddition reactions. | ||
Numerical experiments
Molecular dynamics simulations were carried out by employing the CHARMM27 (ref. 11) force field and the parallel GROMACS4.5.5 simulation package.12 The cell membrane comprised 8580 dipalmitoylphosphatidylcholine (DPPC) lipid residues and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 water molecules,13 building up 106
296 water molecules,13 building up 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 776 atom structures in total. To investigate the effect of the chirality and identical length, end-capped SWCNTs with (5, 5), (10, 0) and (10, 10) chiral indices were generated with 1300, 1500 and 2600 carbon atoms, respectively. The individual nanotubes were placed on top of the lipid bilayer membrane at a 2 Å clearance aligned perpendicular to the membrane upper surface. To probe the effect of the surface functionalization, 36 PEG single chain molecules (n = 1) (35 atoms per chain) were covalently attached to the SWCNTs via carboxylic acid groups (Fig. 1a). All the SWCNTs in the simulations maintained the same electrostatic charge and a fixed PEG density for the functionalized-SWCNTs (f-SWCNTs). Fig. 2 shows the relative positions and density of the PEG ligands attached to a (10, 0) SWCNT which was geometrically relaxed to reach the minimum potential energy level and hence the most stable configuration.
776 atom structures in total. To investigate the effect of the chirality and identical length, end-capped SWCNTs with (5, 5), (10, 0) and (10, 10) chiral indices were generated with 1300, 1500 and 2600 carbon atoms, respectively. The individual nanotubes were placed on top of the lipid bilayer membrane at a 2 Å clearance aligned perpendicular to the membrane upper surface. To probe the effect of the surface functionalization, 36 PEG single chain molecules (n = 1) (35 atoms per chain) were covalently attached to the SWCNTs via carboxylic acid groups (Fig. 1a). All the SWCNTs in the simulations maintained the same electrostatic charge and a fixed PEG density for the functionalized-SWCNTs (f-SWCNTs). Fig. 2 shows the relative positions and density of the PEG ligands attached to a (10, 0) SWCNT which was geometrically relaxed to reach the minimum potential energy level and hence the most stable configuration.
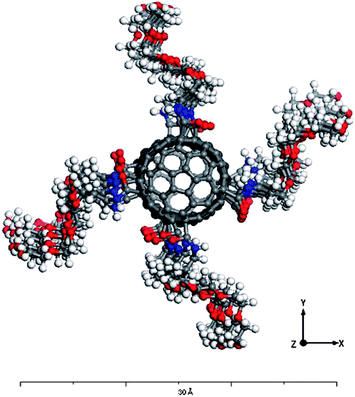 | ||
| Fig. 2 A top view of the generated structure for a (10, 0) end-capped f-SWCNT. The PEG chains were spaced equally along the CNT circumference. | ||
The constructed systems were geometrically relaxed by minimizing their potential energy via the steepest decent minimization method. Molecular dynamics simulations were then carried out under a canonical (NVT)14 thermodynamic ensemble to best describe the in vivo environment of living cells for 100 ps with a 1 fs integration time step. The temperature convergence to the pre-set physiological temperature of 320 K was monitored using a Berendsen thermostat.15
Results and discussion
Fig. 3 depicts the potential energy per atom of each configuration calculated via the steepest decent minimization algorithm. Adding the PEG functionalizing ligands increased the energy per atom of the system for the as constructed system and they maintained their higher energy after the minimization as well.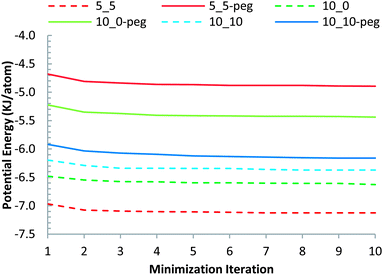 | ||
| Fig. 3 Steepest descent energy minimization data for different configurations. | ||
It is worth noting that, while the SWCNT with the smallest diameter, with (5, 5) chirality, possessed the lowest potential energy, upon functionalizing it with PEG, the f-SWCNT with (5, 5) chirality attained the highest potential energy. In comparison, the highest chiral nanotube simulated, the (10, 10) SWCNT system did not exhibit a large potential energy increase upon functionalizing it with the PEG chains.
The geometrically optimized SWCNT–membrane and f-SWCNT–membrane systems were further equilibrated by running molecular dynamics utilizing the NVT statistical thermodynamics ensemble and equilibrating the whole system with an external thermal bath at 320 K. Fig. 4 shows both the total (potential + kinetics) energy per atom of the systems as well as their temperature evolutions during the 100 ps MD simulations. The energy plots for different systems reflect relatively stabilized regimes with a narrow variation interim after almost 50 ps where the convergence criterion of 0.01 kcal per atom2 was attained.
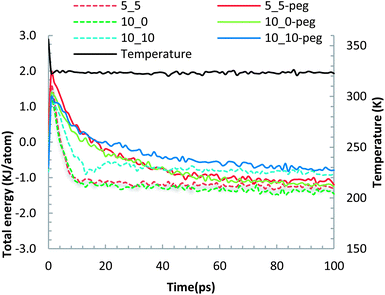 | ||
| Fig. 4 The evolutions of the normalized total energy and temperature for different CNT–membrane configurations during 100 ps of molecular dynamics. | ||
The trajectories of the different configurations at different time intervals are investigated to probe the governing mechanism(s) of the SWCNT and f-SWCNT internalization through the bilayer lipid membrane. The trajectories of different SWCNTs at 5, 10, 15, 50 and 100 ps are plotted in Fig. 5. The trajectory snapshots substantiate the chirality-dependence of SWCNT penetration which confirms our findings in a previous investigation for a much smaller CNT–lipid system at considerably shorter MD durations.2 The tubes with lower chiral indices find their way through the bilayer lipid faster for the given length and electrostatic charge. This is identical to the reported behaviour of SWCNTs with different chiralities but made of an equal number of atoms.2
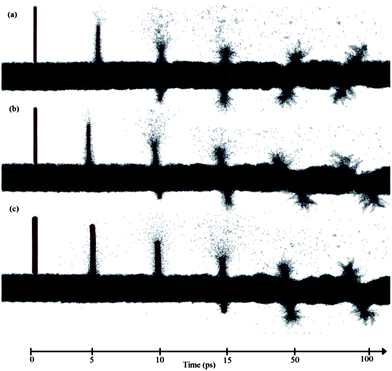 | ||
| Fig. 5 Trajectories of different SWCNTs at selected MD time instances. The snapshots reveal the penetration into the lipid membrane by (a) (5, 5), (b) (10, 0) and (c) (10, 10) SWCNTs. | ||
Fig. 6 illustrates another set of MD snapshots at 30, 40, 45, 50, and 100 ps from the f-SWCNT systems penetrating through the lipid membrane. Comparing the SWCNT and f-SWCNT with identical chiralities highlights the fundamental differences in their internalization phenomena. Both the SWCNTs and f-SWCNTs embrace multiple mechanisms to find their way through the lipid membrane; penetration at the early stages turns into endocytosis dominated insertion at later stages of the MD.
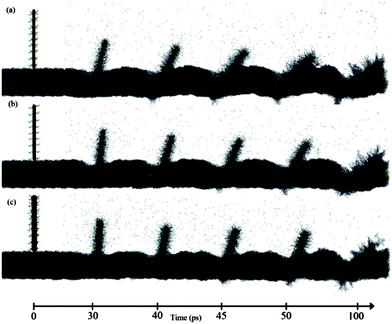 | ||
| Fig. 6 Trajectories of different f-SWCNTs at selected MD time instances. The snapshots reveal the penetration into the lipid membrane by (a) (5, 5), (b) (10, 0) and (c) (10, 10) f-SWCNTs. | ||
Comparing Fig. 5 and 6 sheds some light on the infiltration mechanism(s) of SWCNTs vs. f-SWCNTs. It can be recognized that a SWCNT tends to have more penetration-like internalization whereas the f-SWCNT follows a prevailing endocytosis path. Less membrane destruction and rather more bending and stretching are imposed on the membrane by the f-SWCNTs in comparison to their pure SWCNT counterparts. The enhancement of penetration due to the existence of hydrophilic coatings was reported previously.9 The tendency towards more bending and stretching of the membrane by the f-SWCNTs can be attributed to two factors. First, the PEG ligands act as physical obstacles that slow down the SWCNT penetration and make the probe bulkier and blunter. Second, the hydrophilic functionality of PEGs modifies the surface properties of the probe in such a way that less segregation occurs to the lipids so that they can maintain their surface tension and stretch considerably without rupture.
For the sake of contrasting the different SWCNT penetration kinematics, Fig. 7 shows the penetration depth (z-location of the bottommost carbon atom) for each SWCNT at different MD simulation times. It can be seen that the elapsed time for the f-SWCNTs to complete their transfection process is considerably longer than that for the SWCNTs with identical chirality. This observation simply implies that the functionalization did not change the chirality-dependent uptake of the SWCNT into the lipid cell membrane. On the other hand, Fig. 7 suggests that the fast insertion of the SWCNTs is followed by a bounce back, while the f-SWCNTs exhibit little to no rebound and significantly shallower penetration depths.
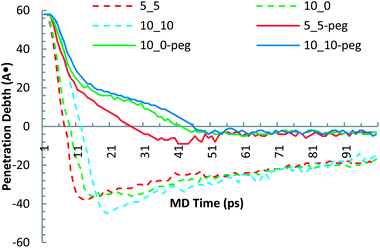 | ||
| Fig. 7 Molecular dynamic evolution of the Z-coordinate trajectory of the bottommost carbon atom for different SWCNT–membrane systems. | ||
Adhesion
As mentioned earlier, the importance of functionalizing the SWCNTs mostly stems from the fact that the opsonins attack the pathogens simply by adhering to them. Hence, investigating the adhesion energy of the SWCNTs to the membrane can disclose valuable information about the mechanism(s) by which a functionalizing agent can assist therapeutic nanoparticles to maintain a high blood circulation lifetime. The adhesion energy is calculated as the difference between the potential energy of the SWCNT–membrane or f-SWCNT–membrane system and the sum of the potential energies of the membrane and the corresponding SWCNT/f-SWCNT: ΔE = Etotal − (ECNT + EM), where Etotal is the total potential energy of the whole system at the end of the MD simulations and ECNT and EM are the potential energies of the SWCNT/f-SWCNT and the membrane, respectively. Separate 100 ps MD simulations were conducted for all the SWCNTs, f-SWCNTs and the membrane under the NVT ensemble to calculate the proper ECNT & EM values for each case. The calculated adhesion energies are plotted in Fig. 8. It can be concluded that SWCNTs with higher chiralities end up with less adhesion energy when functionalized with a small number of PEG chains. However, the adhesion energy associated with the SWCNT with the lowest chirality (i.e. (5, 5)) tends not to be affected with PEG functionalization. Actually, an inverse relationship between the chirality and the adhesion energy was reported for systems incorporating SWCNTs and polyethylene as well.16 The 0.79% increase for the case of (5, 5) chirality can be neglected due to the randomness of the initial velocities in the first step of the dynamic run. These findings are in good agreement with the published experimental observation where PEGs helped the nanoparticles to circulate more and easier in the blood without being attacked by blood cells.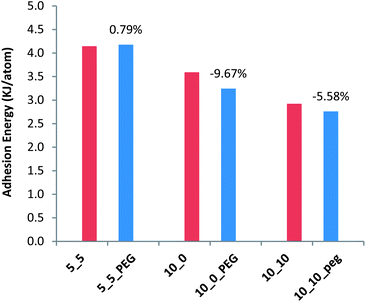 | ||
| Fig. 8 Changes in the adhesion energy due to PEG functionalization of different SWCNTs. Red bars represent the adhesion energy for SWCNTs with different chiralities with the membrane and the adjacent blue bars represent the adhesion energy for PEG f-SWCNTs with the membrane. | ||
Conclusions
The MD simulations revealed that the presence of the (PEG) molecules affects the SWCNT penetrability into the lipid membrane, supporting an earlier experimental investigation by the authors.3a Furthermore, the nanotubes with higher chiral indices penetrate the membrane at a slower rate regardless of the surface functionalization with PEG chains which reveals the marginal contribution of PEG (n = 1) to the potential energy of the overall system. The molecular dynamics simulations divulged a significant difference in the penetration mechanisms of f-SWCNTs versus SWCNTs where the PEGylated ones tend to penetrate with a less energy-dependent mechanism inflicting more deflection to the membrane. It was shown that the presence of a small percentage of water soluble PEG chains, attached covalently to the SWCNT, can slow down the penetration process irrespective of the chirality. This property can be utilized for employing SWCNTs in a more controllable selective targeting of desired cells.Finally, the PEG chains reduced the adhesion energy up to 10% which concurs with the experimental findings about the circulation time of PEGylated particles in the blood serum.
Acknowledgements
The authors would like to thank Dr Justin A. Lemkul, a research scientist in the Department of Biochemistry at Virginia Tech for his invaluable comments on running and troubleshooting GROMACS.Notes and references
- (a) P. Cherukuri, S. M. Bachilo, S. H. Litovsky and R. B. Weisman, Nearinfrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells, J. Am. Chem. Soc., 2004, 126(48), 15638–15639 CrossRef CAS PubMed; (b) N. Kam, T. Jessop, P. Wender and H. Dai, Nanotube molecular transporters: internalization of carbon nanotubeprotein conjugates into mammalian cells, J. Am. Chem. Soc., 2004, 126(22), 6850–6851 CrossRef CAS PubMed; (c) Q. Lu, J. M. Moore, G. Huang, A. S. Mount, A. M. Rao, L. L. Larcom and P. C. Ke, RNA Polymer Translocation with Single-Walled Carbon Nanotubes, Nano Lett., 2004, 4(12), 2473–2477 CrossRef CAS; (d) D. Pantarotto, J. Briand, M. Prato and A. Bianco, Translocation of bioactive peptides across cell membranes by carbon nanotubes, Chem. Commun., 2004, 16–17 RSC; (e) W. Tian and Y. Ma, Theoretical and computational studies of dendrimers as delivery vectors, Chem. Soc. Rev., 2013, 42, 705 RSC.
- A. A. Skandani, R. Zeineldin and M. Al-Haik, Effect of chirality and length on the penetrability of single-walled carbon nanotubes into lipid bilayer cell membranes, Langmuir, 2012, 28(20), 7872–7879 CrossRef CAS PubMed.
- (a) R. Zeineldin, M. Al-Haik and L. G. Hudson, Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells, Nano Lett., 2009, 9(2), 751–757 CrossRef CAS PubMed; (b) M. Bottini, N. Rosato and N. Bottini, PEG-modified carbon nanotubes in biomedicine: current status and challenges ahead, Biomacromolecules, 2011, 12(10), 3381–3393 CrossRef CAS PubMed.
- A. Bianco, J. Hoebeke, S. Godefroy, O. Chaloin, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and C. D. Partidos, Cationic Carbon Nanotubes Bind to CpG Oligodeoxynucleotides and Enhance Their Immunostimulatory Properties, J. Am. Chem. Soc., 2005, 127(1), 58–59 CrossRef CAS PubMed.
- A. A. Bhirde, S. Patel, A. A. Sousa, V. Patel, A. A. Molinolo, Y. Ji, R. D. Leapman, J. S. Gutkind and J. F. Rusling, Distribution and clearance of PEG–single-walled carbon nanotube cancer drug delivery vehicles in mice, Nanomedicine, 2010, 5(10), 1535–1546 CrossRef CAS PubMed.
- S. M. Ryan, G. Mantovani, X. Wang, D. M. Haddleton and D. J. Brayden, Advances in PEGylation of important biotech molecules: delivery aspects, Expert Opin. Drug Delivery, 2008, 5, 371–383 CrossRef CAS PubMed.
- P. N. Yaron, B. D. Holt, P. A. Short, M. Losche, M. F. Islam and K. N. Dahl, Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration, J. Nanobiotechnol., 2011, 9, 45 CrossRef CAS PubMed.
- (a) H. Lee, Interparticle dispersion, membrane curvature, and penetration induced by single-walled carbon nanotubes wrapped with lipids and PEGylated lipids, J. Phys. Chem. B, 2013, 117(5), 1337–1344 CrossRef CAS PubMed; (b) S. Kraszewski, A. Bianco, M. Tarek and C. Ramseyer, Insertion of short amino-functionalized single-walled carbon nanotubes into phospholipid bilayer occurs by passive diffusion, PLoS One, 2012, 7(7), e40703 CAS; (c) V. K. Gangupomu and F. M. Capaldi, Interactions of Carbon Nanotube with Lipid Bilayer Membranes, J. Nanomater., 2011, 2011, 1–6 CrossRef PubMed.
- F. Liu, D. Wu, R. D. Kamm and K. Chen, Analysis of nanoprobe penetration through a lipid bilayer, Biochim. Biophys. Acta, 2013, 1828(8), 1667–1673 CrossRef CAS PubMed.
- A. Z. Wang, F. Gu, L. Zhang, J. M. Chan, A. Radovic-Moreno, M. R. Shaikh and O. C. Farokhzad, Biofunctionalized targeted nanoparticles for therapeutic applications, Expert Opin. Biol. Ther., 2008, 8(8), 1063–1070 CrossRef CAS PubMed.
- S. E. Feller, D. Alexander and J. MacKerell, An Improved Empirical Potential Energy Function for Molecular Simulations of Phospholipids, J. Phys. Chem. B, 2000, 104, 7510–7515 CrossRef CAS.
- (a) B. Hess, C. Kutzner, D. V. D. Spoel and E. Lindahl, GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation, J. Chem. Theory Comput., 2008, 4, 435–447 CrossRef CAS; (b) H. J. C. Berendsen, D. V. D. Spoel and R. V. Drunen, GROMACS: a message-passing parallel molecular dynamics implementation, Comput. Phys. Commun., 1995, 91, 43–56 CrossRef CAS; (c) D. V. D. Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark and H. J. Berendsen, GROMACS: fast, flexible, and free, J. Comput. Chem., 2005, 26(16), 1701–1718 CrossRef PubMed.
- S. Miyamoto and P. A. Kollman, An Analytical Version of the SHAKE and RATTLE Algorithms for Rigid Water Models, J. Comput. Chem., 1992, 13, 952–962 CrossRef CAS.
- C. A. Hans, Molecular dynamics simulations at constant pressure and/or temperature, J. Chem. Phys., 1980, 72, 2384–2393 CrossRef.
- H. J. C. Berendsen, J. P. M. Postma, W. F. V. Gunsteren, A. DiNola and J. R. Haak, Molecular dynamics with coupling to an external bath, J. Chem. Phys., 1984, 81, 3684 CrossRef CAS.
- M. S. Al-Haik and M. Y. Hussaini, Adhesion energy of single-wall carbon nanotube–polyethylene composite: effect of chirality, J. Appl. Phys., 2005, 97, 074306 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
