 Open Access Article
Open Access ArticleMonolayer spontaneous curvature of raft-forming membrane lipids†
Benjamin Kollmitzera, Peter Heftbergera, Michael Rappoltbc and Georg Pabst*a
aInstitute of Molecular Biosciences, Biophysics Division, University of Graz, Austria. E-mail: Georg.Pabst@uni-graz.at; Fax: +43 316 4120-390; Tel: +43 316 4120-342
bInstitute of Inorganic Chemistry, Graz University of Technology, Austria
cSchool of Food Science and Nutrition, University of Leeds, UK
First published on 27th September 2013
Abstract
Monolayer spontaneous curvatures for cholesterol, DOPE, POPE, DOPC, DPPC, DSPC, POPC, SOPC, and egg sphingomyelin were obtained using small-angle X-ray scattering (SAXS) on inverted hexagonal phases (HII). Spontaneous curvatures of bilayer forming lipids were estimated by adding controlled amounts to a HII forming template following previously established protocols. Spontaneous curvatures of both phosphatidylethanolamines and cholesterol were found to be at least a factor of two more negative than those of phosphatidylcholines, whose J0 values are closer to zero. Interestingly, a significant positive J0 value was retrieved for DPPC. We further determined the temperature dependence of the spontaneous curvatures J0(T) in the range from 15 to 55 °C, resulting in a quite narrow distribution of −1 to −3 × 10−3 (nm °C)−1 for most investigated lipids. The data allowed us to estimate the monolayer spontaneous curvatures of ternary lipid mixtures showing liquid ordered/liquid disordered phase coexistence. We report spontaneous curvature phase diagrams for DSPC/DOPC/Chol, DPPC/DOPC/Chol and SM/POPC/Chol and discuss effects on protein insertion and line tension.
1 Introduction
Curvature is an essential ingredient in a cell's life and occurs most visibly during membrane fusion and fission processes, e.g. exocytosis and endocytosis, or when a cell is attacked by an enveloped virus.1 Such events may be induced by proteins, but are also known to depend strongly on the molecular properties of the constituent membrane lipids.2 For instance membrane fusion can take place in the absence of proteins.3 Lipid-driven membrane curvature may result e.g. from unequally distributed lipids of the same type in the opposing membrane leaflets or from asymmetric distributions of lipids with different molecular shapes due to their different intrinsic curvatures.4–9In general, lipids with molecular shapes different from cylinders will form monolayers that either curve away or towards the polar/apolar interface.10 In planar membranes, however, such monolayers are forced into a flat topology, where they lie back-to-back – in order to avoid energetically unfavorable voids – leading to significant curvature elastic stress that is stored within the membrane. This elastic stress may have several functional consequences for membranes and can be viewed as a hidden dimension of membrane curvature. Of particular interest is the role of intrinsic/spontaneous curvature in coupling to protein function11–18 and in determining the line tension of lipid domains mimicking membrane rafts.19,20
As per definition the spontaneous curvature J0 = 0 for cylindrically formed lipids, J0 < 0 for lipids with tail regions of bigger lateral cross-section than the headgroups and vice versa for J0 > 0. For example, lipids with a negative spontaneous curvature are prone to form non-planar structures like inverted hexagonal phases HII. More precisely the radius of curvature of an unstressed monolayer at its neutral plane equals 1/J0.21,22 The neutral plane is defined as the position at which bending and stretching modes are decoupled, i.e. bending and stretching deformations proceed independently from each other.23 A second, frequently quoted surface within the monolayer of amphiphiles is the pivotal plane, which occurs where the molecular area does not change upon deformation. Pioneered by the groups of Rand and Gruner during the late 80s and the 90s, the position of this surface and consequently the spontaneous curvature at the pivotal plane J0p have been determined to high accuracy for a couple of membrane lipids,21,24–30 for review see ref. 31. The basic idea of these experiments is to use HII phases, where the lipid monolayers expose their intrinsic curvature within the individual rods and to determine the pivotal plane by bending and compressing the rods either by gravimetric dehydration or application of osmotic pressure, while measuring the crystalline lattice via X-ray scattering. For a limited number of lipids the neutral plane has been estimated from the pivotal surface using area compressibility and bending rigidity data.21,23,32
In the present work we determine J0 under stress-free conditions by locating the neutral plane from electron density maps of HII phases. In particular we focus on spontaneous curvature data of lipids which are involved in the formation of membrane rafts. Such data are especially of need for calculating protein partitioning in diverse lipid environments11–18 or to estimate the line-tension of lipid domains.19,20 Additionally, the temperature dependence of spontaneous curvature is still barely investigated. We intend to bridge this gap by determining J0 for cholesterol, DOPC, DPPC, DSPC, POPC, SOPC and egg sphingomyelin within a DOPE matrix from 15 to 55 °C and for POPE at 37 and 55 °C.
2 Materials and methods
2.1 Sample preparation
Cholesterol (Chol), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC), and chicken egg sphingomyelin (eggSM) were purchased from Avanti Polar Lipids, Inc., Alabaster, AL, USA and used without further purification. 9-cis-Tricosene was obtained from Sigma-Aldrich, Austria.After weighing, lipids were dissolved in chloroform–methanol 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a concentration of 10 mg ml−1. These lipid stock solutions were mixed in glass vials, 12 wt% tricosene was added and the organic solvent was evaporated under a gentle nitrogen stream. To remove the remaining solvent, the samples were placed in a vacuum overnight. 18 MΩ cm−1 water (UHQ PS, USF Elga, Wycombe, UK) was added to 20 μl mg−1 lipid and the mixtures with repeated freeze–thaw cycles fully hydrated. The samples were then protected against oxidation with argon, the vials closed and taped, and stored at 4 °C for 6–7 days until the measurement.
1 at a concentration of 10 mg ml−1. These lipid stock solutions were mixed in glass vials, 12 wt% tricosene was added and the organic solvent was evaporated under a gentle nitrogen stream. To remove the remaining solvent, the samples were placed in a vacuum overnight. 18 MΩ cm−1 water (UHQ PS, USF Elga, Wycombe, UK) was added to 20 μl mg−1 lipid and the mixtures with repeated freeze–thaw cycles fully hydrated. The samples were then protected against oxidation with argon, the vials closed and taped, and stored at 4 °C for 6–7 days until the measurement.
2.2 X-ray measurements
Small-angle X-ray scattering (SAXS) was performed at the Austrian SAXS beamline at ELETTRA, Trieste.33,34 A mar300 Image Plate 2D detector from Marresearch, Norderstedt, Germany was used covering a q-range from 0.2–6.1 nm−1 and calibrated with silver-behenate (CH3(CH2)20COOAg) with a d-spacing of 5.838 nm.35 Sample temperatures were controlled with a bath thermostat from Huber, Offenburg, Germany to a precision of ±0.1 °C. The samples were equilibrated for 10 min at given temperatures before exposure. The exposure time was set to 30 s.2.3 X-ray data analysis
Image integration was performed with FIT2D36,37 and cross-checked with MATLAB®.38 For further data analysis, homemade MATLAB scripts were used and their function verified with FIT2D,39 IDL®,40 and IGOR Pro®.41Standard procedures were used to determine the lattice parameters and calculate electron-density maps of the HII (for further details, see S1 of the ESI†). In brief, we applied Lorentzians and additive linear background estimators to fit the Bragg peaks. Typically 5–7 peaks were discernible in the patterns, although for higher temperatures and some samples only three or four peaks could be detected. This was considered in the uncertainty estimations.
The lattice parameter a was determined via the reflection law, taking into account the information from all Lorentzians. Fourier synthesis yielded the electron density ρ(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) ) in real-space, with the phasing condition (+ − − + + + + + −) known from the literature for DOPE-rich, fully hydrated HII phases.42–44 Other phase combinations were tested, but yielded electron densities incompatible with the known structure.
) in real-space, with the phasing condition (+ − − + + + + + −) known from the literature for DOPE-rich, fully hydrated HII phases.42–44 Other phase combinations were tested, but yielded electron densities incompatible with the known structure.
2.4 Spontaneous curvature estimation
We first locate the position Rp of the lipid headgroup by fitting a Gaussian to a radial section of the electron density map in a region of ∼1 nm around the maximum value (see S1 in the ESI† for further details). Then, the neutral surface is simply given by R0 = Rp + dH1, where dH1 is the distance between the headgroup and the glycerol backbone. Using a joint refinement of X-ray and neutron data on lamellar phases, Kučerka and coworkers reported high-resolution structural data for a series of phospholipids.46–49 The reported dH1 values range between 0.37 and 0.50 nm at temperatures from 20 to 50 °C. We apply the average of these values for our R0 calculations dH1 = (0.44 ± 0.05) nm. To test the applicability of this procedure, we compare J0 = (−0.387 ± 0.011) nm−1 retrieved from the present analysis for DOPE at 25 °C with J0 = (−0.367 ± 0.010) nm−1 estimated from measurements of the pivotal surface.21 A small difference is expected due to the presence of tricosene in the present experiments in order to reduce packing frustration (see Section 2.4.2) as compared to the measurements performed by Leikin et al.21
We also attempted to derive J0 from the width σp of the Gaussian fitted to the headgroup region of the radial electron density profiles, i.e. R0 = Rp + σp. However, the resolution of the electron density maps was too poor for some lipid mixtures, yielding σp > 0.7 nm and hence unrealistic locations of the glycerol backbone.
| Jmix0 = χJguest0 + (1 − χ)Jhost0, | (1) |
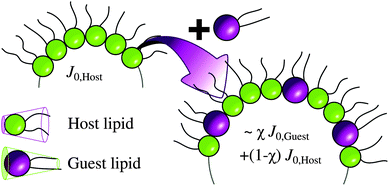 | ||
| Fig. 1 Guest lipid is incorporated at a concentration χ within the host's template phase. Note the change of the curvature upon mixing. | ||
All bilayer-forming lipids were measured at concentrations of 10, 20, 30, 40 and 50 mol% in DOPE. The extrapolation according to eqn (1) was performed using all concentrations below a critical value χcrit, at which:
• immiscibility was directly observed because non-hexagonal Bragg peaks were visible,
• eqn (1) did not obviously hold anymore, or
• the lattice parameter a did not change smoothly with χ.
Entropic contributions get more pronounced at higher temperatures, which generally leads to improved miscibilities. Accordingly, we observed a monotonic increase of χcrit with T for all samples. An example of the occurrence of non-hexagonal peaks is given in Fig. 2.
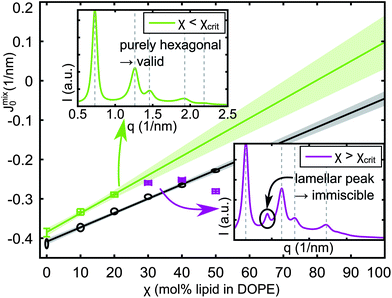 | ||
| Fig. 2 Determination of JDPPC0 at 25 °C (crosses) and JPOPC0 at 45 °C (ellipses) by extrapolation of Jmix0 towards χ = 100%. The insets show X-ray patterns for the last valid (top left) and the first immiscible DPPC data points (bottom right). | ||
Good miscibility was observed for Chol and all unsaturated lipids. For saturated lipids χcrit was not equally satisfactory, but improved above the melting transition of the guest lipid with the exception of eggSM, where only 10 mol% could be incorporated into the DOPE matrix at all temperatures. The number of useful data points (where χ < χcrit) is taken into account for determining the uncertainty of the resulting J0. Extrapolation plots and χcrit (T) for all lipids are reported in S4 of the ESI.†
| J0(T) = k(T − Tm) + J0m | (2) |
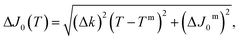 | (3) |
3 Results
Chol, DOPC, DPPC, DSPC, POPC, SOPC and eggSM were mixed with DOPE and measured as detailed in the previous section. The pure lipids' monolayer spontaneous curvatures for each temperature were obtained by eqn (1) (data in S4 of the ESI†). Linear fits of the temperature dependence of J0 yielded the values listed in Table 1 (fits in S5 of the ESI†). By inserting these parameters in eqn (2) and (3), J0 and its uncertainty are readily available for any temperature from 15 to 55 °C.| Lipid | J0m ± ΔJ0m (1 nm) | k ± Δk (10−3/nm °C) |
|---|---|---|
| DOPE | −0.399 ± 0.005 | −1.3 ± 0.4 |
| POPE (*) | −0.316 ± 0.007 | −2.7 ± 0.7 |
| Chol | −0.494 ± 0.013 | −3.5 ± 0.9 |
| DOPC | −0.091 ± 0.008 | −1.1 ± 0.6 |
| DPPC | +0.068 ± 0.032 | −3.5 ± 2.3 |
| DSPC | −0.100 ± 0.044 | −0.2 ± 3.4 |
| POPC | −0.022 ± 0.010 | −1.8 ± 0.7 |
| SOPC | −0.010 ± 0.018 | −2.2 ± 1.3 |
| eggSM | −0.134 ± 0.072 | +1.4 ± 5.1 |
POPE was measured with 12 wt% tricosene and excess water at 37 and 55 °C in the absence of DOPE. The slope and offset of a straight line through the two points following eqn (2) with Tm = 37 °C are given in Table 1.
Fig. 3 compares our results for cholesterol with literature data.‡ Although it seems like the literature data has a positive slope of J0(T), this is probably a coincidence and due to the uncorrelated experiments in different lipid host systems. Generally, one would expect the chains to be more flexible and therefore also occupy more space at higher temperature, corresponding to a more negative spontaneous curvature. This behavior corresponds to k < 0, which is the case for all lipids except for eggSM. This is most likely an artifact due to the limited miscibility of eggSM with DOPE. The limited miscibility also affected other saturated lipids leading to significant experimental uncertainties in k. The overall k varied in a quite narrow window from −1 to −3.5 × 10−3 (nm °C)−1, cf.Table 1, in good agreement with k = (−1.7 ± 0.3) × 10−3 (nm °C)−1, reported for DOPE at temperatures from 15 to 30 °C.27
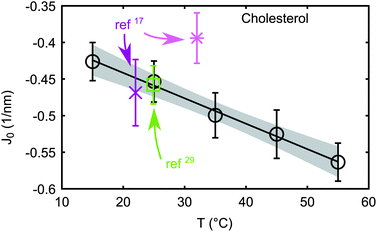 | ||
| Fig. 3 Comparison between cholesterol spontaneous curvature from the literature (ref. 17 and 29) and new data (circles). The straight line corresponds to linear fit. Literature data at 32 °C have been determined in a DOPC host matrix, and the other two in DOPE. | ||
Interestingly, DPPC is the only bilayer-forming lipid with a significant positive J0. DSPC, for example, with the same headgroup but longer chains has J0 = −0.1 nm−1 at 35 °C. Thus, the headgroup contribution to the molecular shape dominates the cross-sectional area and hence J0 of DPPC, whereas chain contributions dominate in the case of DSPC. Mismatch in lateral areas of heads and chains is known to cause chain tilt and the ripple phase for saturated phosphatidylcholines in a certain range of chain lengths.60 Surprisingly, J0 ∼ −0.1 nm−1 also for eggSM, which similar to PCs has a choline moiety in the headgroup and is predominantly composed of the same hydrocarbons as DPPC. Here the sphingosine backbone of eggSM seems to make the difference by taking up more lateral space than the glycerol backbone of PCs. A detailed investigation of this effect is, however, beyond the scope of the present work.
4 Discussion
4.1 Monolayer spontaneous curvature of phase separated systems
For known compositions, monolayer spontaneous curvatures of mixtures are readily computable by generalization of eqn (1) to more components, resulting in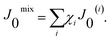 | (4) |
As already mentioned, miscibility is required for the linear additivity of spontaneous curvatures. We assume that this criterion is fulfilled within individual domains of a phase separated system, i.e. non-ideal mixing is not considered. Thus if the compositions of coexisting phases are known, eqn (4) can be applied to determine their spontaneous curvatures. In the case of non-ideal mixing, which may occur for example by a preferred location of lipids at the domain boundary, energetic contributions from lipid–lipid interactions and mixing entropies need to be considered (see e.g.ref. 58). However, this is beyond the scope of the present paper.
Compositional phase diagrams including tielines have been published recently for ternary lipid mixtures exhibiting liquid disordered (Ld)/liquid ordered (Lo) phase coexistences.61–63 These mixtures are simple lipid-only models for membrane rafts, complex platforms which are thought to enable cellular communication and material transport.64 We parameterized the proposed coexistence regions and tieline fields according to the method introduced by Smith and Freed65 and slightly modified by Heberle et al.,62 whose notation we adopted. Briefly, a given phase coexistence region is approximated via a Bézier curve of degree five, while a single variable takes care of the tieline fanning. The parameter u ∈ [0, 1] identifies a particular tieline, with the critical point (tieline of length 0) at u = 0 and the tieline farthest away from the critical point at u = 1. More details on this parameterization and the explicit values can be found in S2 of the ESI.†
Fig. 4 compares the spontaneous curvatures for coexisting Lo/Ld phases. The mixture POPC/eggSM/Chol behaves as expected, i.e. due to the negative intrinsic curvature of cholesterol, the Lo phase, which contains about twice as much cholesterol as Ld domains, exhibits a more negative J0. Also DOPC/DSPC/Chol shows a similar behaviour, although the measurement uncertainty limits a clear distinction of the spontaneous curvatures of Lo and Ld. For DOPC/DPPC/Chol, however, J0 of the liquid ordered phase at high values of u is less negative than for the Ld phase, and within the measurement uncertainty it could even be slightly positive. This results from a more positive J0 of DPPC as compared to DSPC with J0 ∼ −0.1 nm−1 (Table 1). We note that the quantitative difference between monolayer spontaneous curvatures of Lo and Ld depends on the exact location of the coexistence region and the tieline orientation, which both contain some uncertainties.
 | ||
| Fig. 4 Spontaneous curvature J0 (white contours and false-color) for three ternary mixtures within the phase diagrams taken from ref. 61–63. White segments are two-phase coexistence regions with tielines, gray triangles are three-phase coexistence regions, and gray stars are critical points (top row). The spontaneous curvature J0 is plotted for coexisting Lo/Ld phases along the boundary of the fluid–fluid phase coexistence regime (bottom row) parameterized by u (see text). | ||
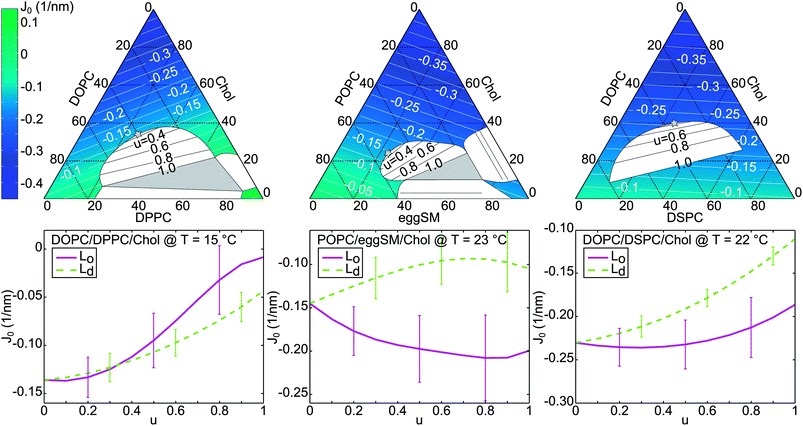
It is instructive to consider the effects of these J0 differences on the insertion probability of simple membrane proteins. Barrel-like transmembrane proteins, which have a thicker cross-section at the center of the bilayer than near the bilayer-water interface, would generally prefer phases with positive spontaneous curvatures, where the effective lipid cross-section at the tail region is smaller than for the headgroup (Fig. 5). In the DOPC/DPPC/Chol case, this simple argument would mean that the Lo phase is more attractive for such proteins. However, a lower-order expansion of the lateral pressure profile already reveals a dependence of protein partitioning on further elastic parameters, specifically bending elasticities and Gaussian curvature moduli of Lo and Ld.11,12 The literature suggests furthermore hydrophobic mismatch66 and disturbance of lipid packing67,68 as important factors for determining protein-insertion energies in membranes. The treatment of these effects is beyond the scope of the present work.
 | ||
| Fig. 5 Barrel shaped transmembrane protein within a bilayer composed of lipids with negative (left) and positive (right) monolayer spontaneous curvatures. For the latter scenario, the protein shape reduces the packing frustration within the bilayer. | ||
4.2 Line tension calculation
Another parameter that is affected by J0 is the line tension γ between two coexisting phases, which influences the size and shape of domains.69,70 Theory predicts an elastic contribution to γ by the monolayer bending moduli, tilt moduli, and thickness difference of Lo/Ld domains (γel) and a second term γJ0, which includes contributions from the spontaneous curvatures.19 In the following paragraphs, we give results for the line tension of ternary and quaternary lipid mixtures and discuss the effect of J0. Calculation details, lipid compositions of Lo and Ld phases, as well as elastic parameters are given in S3 of the ESI.† It is important to note that Helfrich's definition of spontaneous curvature,71 which has been applied for deriving γJ0 in ref. 19, differs from the quantity J0 which we determine in the present work. However, in the case of linear bending behavior, or for small deviations from a flat monolayer, i.e. if the spontaneous curvature is much smaller than the inverse monolayer thickness h, the two values are approximately equal.22 In S3 of the ESI,† we show that indeed |J0| < 1/h for the following calculations.Just recently, bending and tilt moduli, as well as structural parameters, have been determined with molecular dynamics (MD) simulations supported by SAXS, for two ternary mixtures showing Lo/Ld phase separation.72 By combining this information with our new curvature data, we calculate γ = 1.4 pN for DOPC/DPPC/Chol and γ = 1.6 pN for DOPC/DSPC/Chol at given Lo/Ld compositions. These values are in the typical range reported from either experiment or theory (see, e.g.ref. 73−76). Because of the positive curvature of DPPC, J0 values for both phases of DOPC/DPPC/Chol are close to zero, leading to vanishing contributions of γJ0 to the line tension. For DOPC/DSPC/Chol, however, the Lo and Ld phases feature a negative J0, leading to γJ0 = −1.8 pN, i.e. the line tension between the coexisting domains is decreased due to the contribution of J0.
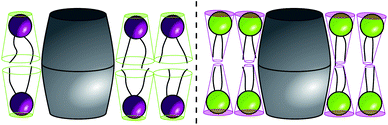
The same theory has been applied to rationalize the transition from nanoscopic to microscopic domains, recently reported for the quaternary mixture DOPC/POPC/DSPC/Chol.77 Starting from nanometer sized domains in POPC/DSPC/Chol, replacing POPC with DOPC has lead to increasing domain sizes, and finally to domains in the micrometer regime for DOPC/DSPC/Chol. Parameterized by the ratio ρ = χDOPC/(χDOPC + χPOPC), the original calculation of the line tension has explained this behavior; but apart from information on the bilayer thickness only estimated values for the parameters influencing γ were available. By applying bending and tilt moduli from MD simulations,72 spontaneous curvatures from the current work, and structural information from Heberle et al.,77 we were able to calculate the line tension for ρ = 1 and give improved estimations for ρ < 1 (Fig. 6). Because of compositional differences for Lo/Ld domains between experiments and MD simulations, the present calculations still rely on considerable assumptions for ρ < 1. In general, the change of nanoscopic to microscopic domains is accompanied by an increase of line tension. This agrees well with our results of γ ∼ 0.5 pN for the nanoscopic regime, γ ∼ 2.5 pN for the microscopic regime, and intermediate in between. The contribution of spontaneous curvature to γ stays nearly constant for all compositions, meaning that the transition from nanoscopic to microscopic domains is mainly driven by bilayer thickness differences in this case, in agreement with the conclusions of the original report.77
 | ||
| Fig. 6 Calculated line tension γ between Lo and Ld domains in DOPC/POPC/DSPC/Chol. Uncertainties of all data points are comparable. | ||
5 Conclusions
By evaluating synchrotron SAXS data of DOPE-rich lipid mixtures in the HII phase, we were able to estimate monolayer spontaneous curvatures J0 for several biologically relevant phospholipids, cholesterol and egg sphingomyelin at temperatures ranging from 15 to 55 °C. Within experimental accuracy, our results are in good agreement with values from more in-depth studies by other groups, conducted at room temperature on DOPE, DOPC, and cholesterol.Our measurements extend the J0-list of lipid species and add their temperature dependence.31 These data will be useful for numerous applications in membrane biophysics.
In the present work we discuss three examples: (i) the monolayer spontaneous curvatures of raft-like lipid mixtures, (ii) line tension of Lo/Ld phases and (iii) evaluation of the line tension during a transition from nanoscopic to microscopic domains. For the studied mixtures of POPC/eggSM/Chol and DOPC/DSPC/Chol, J0 of the Lo phase was found to be more negative than that of the coexisting Ld phase. DOPC/DPPC/Chol however shows a contrary behavior, with a more positively curved liquid ordered phase due to the positive J0 of DPPC. This would favor partitioning of barrel-shaped proteins into the Lo phase. Regarding line tension, we found only significant contributions of J0 for coexisting domains in DOPC/DSPC/Chol. In DOPC/DPPC/Chol and also for the transition from nanoscopic to microscopic domains, γ seems to be dominated by elastic moduli and thickness differences.
Acknowledgements
This work is supported by the Austrian Science Fund FWF, Project no. P24459-B20. The authors thank Karl Lohner, George Khelashvili, Siewert-Jan Marrink, and Ilya Levental for valuable discussions and in particular Daniel Harries for pointing us at the literature explaining delicate differences in spontaneous curvatures.References
- K. N. Burger, Traffic, 2000, 1, 605–613 CrossRef CAS.
- L. V. Chernomordik and M. M. Kozlov, Nat. Struct. Mol. Biol., 2008, 15, 675–683 CAS.
- L. K. Tamm, J. Crane and V. Kiessling, Curr. Opin. Struct. Biol., 2003, 13, 453–466 CrossRef CAS.
- M. P. Sheetz and S. J. Singer, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 4457–4461 CrossRef CAS.
- E. Evans, Biophys. J., 1974, 14, 923–931 CrossRef CAS.
- S. Svetina, A. Ottova-Leitmannová and R. Glaser, J. Theor. Biol., 1982, 94, 13–23 CrossRef CAS.
- S. Svetina and B. Žekš, Eur. Biophys. J., 1989, 17, 101–111 CrossRef CAS.
- B. Bozic, S. Svetina, B. Zeks and R. E. Waugh, Biophys. J., 1992, 61, 963–973 CrossRef CAS.
- L. Miao, U. Seifert, M. Wortis and H.-G. Döbereiner, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1994, 49, 5389–5407 CrossRef CAS.
- J. Seddon and R. Templer, Handbook of biological physics, North-Holland, 1995, vol. 1, pp. 97–160 Search PubMed.
- R. S. Cantor, J. Phys. Chem. B, 1997, 101, 1723–1725 CrossRef CAS.
- R. S. Cantor, Chem. Phys. Lipids, 1999, 101, 45–56 CrossRef CAS.
- S. A. Safran, J. Stat. Phys., 1995, 78, 1175–1177 CrossRef.
- R. Brewster and S. A. Safran, Biophys. J., 2010, 98, L21–L23 CrossRef CAS PubMed.
- J. A. Lundbæk, P. Birn, J. Girshman, A. J. Hansen and O. S. Andersen, Biochemistry, 1996, 35, 3825–3830 CrossRef PubMed.
- D. Marsh, Biophys. J., 2007, 93, 3884–3899 CrossRef CAS PubMed.
- B. Boulgaropoulos, M. Rappolt, B. Sartori, H. Amenitsch and G. Pabst, Biophys. J., 2012, 102, 2031–2038 CrossRef CAS PubMed.
- G. Pabst, B. Boulgaropoulos, E. Gander, B. R. Sarangi, H. Amenitsch, V. A. Raghunathan and P. Laggner, J. Membr. Biol., 2009, 231, 125–132 CrossRef CAS PubMed.
- P. I. Kuzmin, S. A. Akimov, Y. A. Chizmadzhev, J. Zimmerberg and F. S. Cohen, Biophys. J., 2005, 88, 1120–1133 CrossRef CAS PubMed.
- S. A. Akimov, P. I. Kuzmin, J. Zimmerberg and F. S. Cohen, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 011919 CrossRef.
- S. Leikin, M. M. Kozlov, N. L. Fuller and R. P. Rand, Biophys. J., 1996, 71, 2623–2632 CrossRef CAS.
- M. M. Kozlov, Methods in Membrane Lipids, Springer, 2007, pp. 355–366 Search PubMed.
- M. M. Kozlov and M. Winterhalter, J. Phys. II, 1991, 1, 1077–1084 CrossRef CAS.
- S. M. Gruner, V. A. Parsegian and R. P. Rand, Faraday Discuss., 1986, 81, 29–37 RSC.
- M. W. Tate and S. M. Gruner, Biochemistry, 1989, 28, 4245–4253 CrossRef CAS.
- R. P. Rand, N. L. Fuller, S. M. Gruner and V. A. Parsegian, Biochemistry, 1990, 29, 76–87 CrossRef CAS.
- M. M. Kozlov, S. Leikin and R. P. Rand, Biophys. J., 1994, 67, 1603–1611 CrossRef CAS.
- R. P. Rand and N. L. Fuller, Biophys. J., 1994, 66, 2127–2138 CrossRef CAS.
- Z. Chen and R. P. Rand, Biophys. J., 1997, 73, 267–276 CrossRef CAS.
- Z. Chen and R. P. Rand, Biophys. J., 1998, 74, 944–952 CrossRef CAS.
- J. Zimmerberg and M. M. Kozlov, Nat. Rev. Mol. Cell Biol., 2005, 7, 9–19 CrossRef PubMed.
- M. M. Kozlov and M. Winterhalter, J. Phys. II, 1991, 1, 1085–1100 CrossRef CAS.
- H. Amenitsch, M. Rappolt, M. Kriechbaum, H. Mio, P. Laggner and S. Bernstorff, J. Synchrotron Radiat., 1998, 5, 506–508 CrossRef CAS PubMed.
- S. Bernstorff, H. Amenitsch and P. Laggner, J. Synchrotron Radiat., 1998, 5, 1215–1221 CrossRef CAS PubMed.
- T. C. Huang, H. Toraya, T. N. Blanton and Y. Wu, J. Appl. Crystallogr., 1993, 26, 180–184 CrossRef CAS.
- A. P. Hammersley, European Synchrotron Radiation Facility Internal Report ESRF97HA02T, 1997 Search PubMed.
- A. P. Hammersley, S. O. Svensson, M. Hanfland, A. N. Fitch and D. Hausermann, High Pressure Res., 1996, 14, 235–248 CrossRef.
- MATLAB v. 7.12 (R2011a), 2011.
- A. P. Hammersley and C. Riekel, Syn. Rad. News, 1989, 2, 24–26 Search PubMed.
- IDL (Interactive Data Language) v. 6.1.
- IGOR Pro v. 6.2.2.2, 2011.
- D. C. Turner and S. M. Gruner, Biochemistry, 1992, 31, 1340–1355 CrossRef CAS.
- P. E. Harper, D. A. Mannock, R. N. Lewis, R. N. McElhaney and S. M. Gruner, Biophys. J., 2001, 81, 2693–2706 CrossRef CAS.
- M. Rappolt, A. Hodzic, B. Sartori, M. Ollivon and P. Laggner, Chem. Phys. Lipids, 2008, 154, 46–55 CrossRef CAS PubMed.
- S. H. Alley, O. Ces, M. Barahona and R. H. Templer, Chem. Phys. Lipids, 2008, 154, 64–67 CrossRef CAS PubMed.
- N. Kučerka, S. Tristram-Nagle and J. F. Nagle, Biophys. J., 2006, 90, L83–L85 CrossRef PubMed.
- N. Kučerka, S. Tristram-Nagle and J. F. Nagle, J. Membr. Biol., 2006, 208, 193–202 CrossRef PubMed.
- N. Kučerka, J. F. Nagle, J. N. Sachs, S. E. Feller, J. Pencer, A. Jackson and J. Katsaras, Biophys. J., 2008, 95, 2356–2367 CrossRef PubMed.
- N. Kučerka, M.-P. Nieh and J. Katsaras, Biochim. Biophys. Acta, Biomembr., 2011, 1808, 2761–2771 CrossRef PubMed.
- G. L. Kirk and S. M. Gruner, J. Phys., 1985, 46, 761–769 CAS.
- H. Vacklin, B. J. Khoo, K. H. Madan, J. M. Seddon and R. H. Templer, Langmuir, 2000, 16, 4741–4748 CrossRef CAS.
- M. Rappolt, A. Hickel, F. Bringezu and K. Lohner, Biophys. J., 2003, 84, 3111–3122 CrossRef CAS.
- E. E. Kooijman, V. Chupin, N. L. Fuller, M. M. Kozlov, B. de Kruijff, K. N. J. Burger and R. P. Rand, Biochemistry, 2005, 44, 2097–2102 CrossRef CAS PubMed.
- S. A. Safran, P. Pincus and D. Andelman, Science, 1990, 248, 354–356 CAS.
- M. M. Kozlov and W. Helfrich, Langmuir, 1992, 8, 2792–2797 CrossRef CAS.
- S. L. Keller, S. M. Bezrukov, S. M. Gruner, M. W. Tate, I. Vodyanoy and V. A. Parsegian, Biophys. J., 1993, 65, 23–27 CrossRef CAS.
- G. Khelashvili, D. Harries and H. Weinstein, Biophys. J., 2009, 97, 1626–1635 CrossRef CAS PubMed.
- S. May and A. Ben-Shaul, J. Chem. Phys., 1995, 103, 3839 CrossRef CAS.
- M. Gradzielski, D. Langevin, T. Sottmann and R. Strey, J. Chem. Phys., 1997, 106, 8232–8238 CrossRef CAS.
- R. Koynova and M. Caffrey, Biochim. Biophys. Acta, Rev. Biomembr., 1998, 1376, 91–145 CrossRef CAS.
- P. Uppamoochikkal, S. Tristram-Nagle and J. F. Nagle, Langmuir, 2010, 26, 17363–17368 CrossRef CAS PubMed.
- F. A. Heberle, J. Wu, S. L. Goh, R. S. Petruzielo and G. W. Feigenson, Biophys. J., 2010, 99, 3309–3318 CrossRef CAS PubMed.
- I. V. Ionova, V. A. Livshits and D. Marsh, Biophys. J., 2012, 102, 1856–1865 CrossRef CAS PubMed.
- D. Lingwood and K. Simons, Science, 2010, 327, 46–50 CrossRef CAS PubMed.
- A. K. Smith and J. H. Freed, J. Phys. Chem. B, 2009, 113, 3957–3971 CrossRef CAS.
- A. Ben-Shaul, Handbook of biological physics, North-Holland, 1995, vol. 1, pp. 359–401 Search PubMed.
- L. V. Schäfer, D. H. de Jong, A. Holt, A. J. Rzepiela, A. H. de Vries, B. Poolman, J. A. Killian and S. J. Marrink, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1343–1348 CrossRef PubMed.
- J. Domański, S. J. Marrink and L. V. Schäfer, Biochim. Biophys. Acta, Biomembr., 2012, 1818, 984–994 CrossRef PubMed.
- A. J. García-Sáez, S. Chiantia and P. Schwille, J. Biol. Chem., 2007, 282, 33537–33544 CrossRef PubMed.
- D. W. Lee, Y. Min, P. Dhar, A. Ramachandran, J. N. Israelachvili and J. A. Zasadzinski, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 9425–9430 CrossRef CAS PubMed.
- W. Helfrich, Z. Naturforsch., C: J. Biosci., 1973, 693–703 CAS.
- G. Khelashvili, B. Kollmitzer, P. Heftberger, G. Pabst and D. Harries, J. Chem. Theory Comput., 2013, 9, 3866–3871 CrossRef CAS PubMed.
- H. J. Risselada and S. J. Marrink, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17367–17372 CrossRef CAS PubMed.
- A. Tian, C. Johnson, W. Wang and T. Baumgart, Phys. Rev. Lett., 2007, 98, 208102 CrossRef.
- C. Esposito, A. Tian, S. Melamed, C. Johnson, S.-Y. Tee and T. Baumgart, Biophys. J., 2007, 93, 3169–3181 CrossRef CAS PubMed.
- A. R. Honerkamp-Smith, P. Cicuta, M. D. Collins, S. L. Veatch, M. den Nijs, M. Schick and S. L. Keller, Biophys. J., 2008, 95, 236–246 CrossRef CAS PubMed.
- F. A. Heberle, R. S. Petruzielo, J. Pan, P. Drazba, N. Kučerka, R. F. Standaert, G. W. Feigenson and J. Katsaras, J. Am. Chem. Soc., 2013, 135, 6853–6859 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Electron density maps, tieline parameterization, line tension calculations, miscibilities, and temperature dependence of spontaneous curvature. See DOI: 10.1039/c3sm51829a |
| ‡ Reported values for J0p17,29 were rescaled to J0 using J0 ∼ J0p (1 + β), with β = 0.065 ± 0.035 determined in ref. 21. Data reported by Boulgaropoulos et al.17 were additionally corrected from J0p = −0.38 nm−1 to −0.43 nm−1 prior to the scaling due to a flaw in their data analysis. |
| This journal is © The Royal Society of Chemistry 2013 |
