 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alkali-metal-mediated zincation (AMMZn) meets N-heterocyclic carbene (NHC) chemistry: Zn–H exchange reactions and structural authentication of a dinuclear Au(I) complex with a NHC anion†
David R. Armstronga,
Sharon E. Bailliea,
Victoria L. Blaira,
Nicolas G. Chabloza,
Josefina Diezb,
Joaquin Garcia-Alvarezb,
Alan R. Kennedya,
Stuart D. Robertsona and
Eva Hevia*a
aWestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, G1 1XL, UK. E-mail: eva.hevia@strath.ac.uk
bDepartamento de Química Orgánica e Inorgánica, Instituto Laboratorio de Compuestos Organometálicos y Catálisis (Unidad Asociada al CSIC), IUQOEM, Facultad de Química, Universidad de Oviedo, E-33071 Oviedo, Spain
First published on 14th August 2013
Abstract
Merging two evolving areas in synthesis, namely cooperative bimetallics and N-heterocyclic carbenes (NHCs), this study reports the isolation of the first intermediates of alkali-metal-mediated zincation (AMMZn) of a free NHC and a Zn–NHC complex using sodium zincate [(TMEDA)NaZn(TMP)(tBu)2] (1) as a metallating reagent. The structural authentication of (THF)3Na[:C{[N(2,6-iPr2C6H3)]2CHCZn(tBu2)}] (2) and [Na(THF)6]+[tBu2Zn:C{[N(2,6-iPr2C6H3)]2CHCZn(tBu2)}]− (4), resulting from the reactions of 1 with unsaturated free NHC IPr (IPr = 1,3-bis(2,6-di-isopropylphenylimidazole-2-ylidene) and NHC complex ZntBu2IPr (3) respectively demonstrates that in both cases, this mixed-metal approach can easily facilitate the selective C4 zincation of the unsaturated backbone of the NHC ligand. Furthermore, the generation of anionic NHC fragments enables dual coordination through their normal (C2) and abnormal (C4) positions to the bimetallic system, stabilising the kinetic AMMZn intermediates which normally go undetected and provides new mechanistic insights in to how these mixed-metal reagents operate. In stark contrast to this bimetallic approach when NHC-complex 3 is reacted with a more conventional single-metal base such as tBuLi, the deprotonation of the coordinated carbene is inhibited, favouring instead, co-complexation to give NHC-stabilised [IPr·LiZntBu3] (5). Showing the potential of 2 to act as a transfer agent of its anionic NHC unit to transition metal complexes, this intermediate reacts with two molar equivalents of [ClAu(PPh3)] to afford the novel digold species [ClAu:C{[N(2,6-iPr2C6H3)]2CHCAu(PPh3)}] (6) resulting from an unprecedented double transmetallation reaction which involves the simultaneous exchange of both cationic (Na+) and neutral (ZntBu2) entities on the NHC framework.
Introduction
Organozinc reagents constitute one of the most valuable and versatile low polarity organometallic reagents in synthesis, playing a key role in many fundamental organic transformations on the account of their marked soft nucleophilic character and exceptional functional group tolerance.1 Despite their numerous applications, the use of simple zinc reagents (alkyls, amides) in deprotonative metalation chemistry has been paltry due to their sluggish kinetic reactivity.2 Notwithstanding, recent advances in bimetallic chemistry have established that this kinetic hurdle can be cleared by pairing zinc reagents with group 1 organometallic compounds to form alkali-metal zincates.3 Operating through metal⋯metal' cooperative effects, these ates often display an enhanced metallating power and special selectivities which allow the direct (one-step) zincation of a wide range of aromatic substrates with a rich variety of functional groups often incompatible with conventional organolithium reagents such as BuLi or LDA.4 Amongst this family of multicomponent reagents, heteroleptic sodium zincate [(TMEDA)NaZn(TMP)(tBu)2] (1) (TMEDA is N,N,N′,N′-tetramethylethylenediamine and TMP is 2,2,6,6-tetramethylpiperidide)5 stands out as a potent reagent capable of executing regioselective mono and dimetallation of non-activated arenes such as benzene6 and naphthalene7 as well as promoting ortho and remarkably meta zincation of substituted arenes.8 By structurally defining the constitution of the organometallic intermediates prior to any electrophilic interception we have demonstrated that these reactions are genuine examples of direct zincation, where heteroleptic zincate 1 displays an overall alkyl basicity.9 Thus, these reactions where the departing hydrogen is replaced by zinc but require the presence of the alkali-metal to succeed are best described as alkali-metal-mediated zincations (AMMZn).5–9Exporting this bimetallic approach into new territory, herein we report the first study in which AMMZn through 1 has been applied to a free N-heterocyclic carbene (NHC) and to a NHC–zinc complex using IPr and IPr·ZntBu2 as case studies (IPr = 1,3-bis(2,6-di-isopropylphenylimidazol-2-ylidene)). Since Arduengo's landmark isolation of the first crystalline NHC,10 an enormous amount of research activity has been devoted to advancing the chemistry of these seminal ligands11 leading to important breakthroughs in transition-metal catalysis.12 Furthermore, recent reports have shown that the use of NHC's in a stabilising role has been crucial for the isolation of highly reactive molecules containing low oxidation state main-group elements.13 Surprisingly, despite their important applications and the fact that modifications on the imidazole backbone of N-heterocyclic carbenes can finely tune their steric and electronic properties,14 it was only 2010 when the first example of selective lithiation at the C4 position of an NHC was documented.15 In this groundbreaking report Robinson et al. describe the synthesis of a novel anionic lithium dicarbene from the reaction of IPr with nBuLi, exhibiting a polymeric arrangement with Li coordination at both the C2 (normal) and C4 (abnormal)16 positions.15,17 The dicarbenic nature of this compound is demonstrated by forming several adducts with group 13 compounds such as AlMe3 and BEt3. Furthermore, the same group has recently shown that this lithium dicarbene can be used as a precursor to prepare NHC-stabilised triorganozincates.18 Although these compounds are prepared using an indirect transmetallating approach, the authors set up the challenge on whether similar compounds could be prepared by the more straightforward direct zincation of an NHC.
Inspired by these intriguing studies and building on our previous work in AMMZn which glimpses the untapped potential this bimetallic approach has to overcome many limitations of traditional lithiation chemistry, in this manuscript, we present our findings on exporting this bimetallic approach into NHC chemistry, providing new mechanistic insights into how these mixed-metal reagents operate as well as establishing a new synthetic tool for the functionalisation of NHC molecules.
Results and discussion
AMMZn reactions of a free NHC and NHC–Zn complex
We started our investigations assessing the metallating ability of 1 by reacting it with free carbene IPr in hexane–THF solvent mixture to afford (THF)3Na[:C{[N(2,6-iPr2C6H3)]2CHCZn(tBu2)}] (2) (crystalline yield, 86%) (Scheme 1a).19 From NMR data in d8-THF solutions (see ESI†), deprotonation of the backbone of the NHC was detected by the large downfield chemical shift of the 4-C resonance in the 13C NMR spectrum (from 122.3 ppm in the free carbene to 159.4 ppm in 2) and the informative singlet at 6.66 ppm for the imidazole CH in the 1H NMR spectrum which lies considerably upfield to that found in IPr (7.19 ppm). Reflecting the loss of symmetry in the imidazole ring, as a consequence of its deprotonation, two distinct sets of Dipp signals are observed in the 1H and 13C NMR spectra of 2. In addition, the appearance of a resonance in the 13C NMR spectra at 201.4 ppm (C2, carbene carbon) established the formation of a NHC-complex (C2 in free IPr resonates at 220.5 ppm).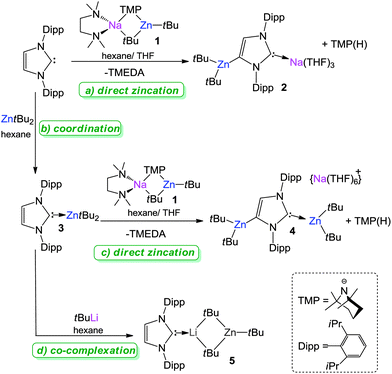 | ||
| Scheme 1 AMMZn of free IPr (a) and Zn–NHC complex 3 (c) by mixed-metal base 1 versus co-complexation reaction of 3 by treatment with homometallic tBuLi (d). | ||
X-ray structure determination of 2 (Fig. 1) confirmed that the C4-metalation of IPr has actually been a zincation. The newly generated anionic carbene acts as an unsymmetrical bridge between the two metals, coordinating through its abnormal C4 position [i.e., C2] to Zn and through its normal C2 position [i.e., C1] to Na, in a contacted ion pair structure which is completed by three THF and two tBu ligands attached to Na and Zn respectively. Interestingly, reflecting the anionic character of the carbene, the Zn–C4 distance in 2 [Zn1–C2, 2.058(3) Å] is noticeably shorter than that found in the normal neutral zinc NHC-complex IPr·ZntBu2 (3) [Zn1–C1, 2.118(5) Å] (see ESI for synthetic and structural details†), being comparable to those found for other α-zincated N-heterocyclic molecules such as N-methylpyrrole [2.0527(18) Å].20
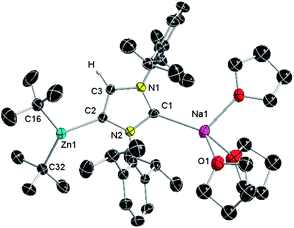 | ||
| Fig. 1 Molecular structure of 2 with 50% probability displacement ellipsoids. Hydrogen atoms, except on the backbone of the imidazole ring, and minor disorder in THF molecules omitted for clarity. | ||
It is worth emphasizing that mainstream alkylzinc reagents fail to promote metalation of IPr, as illustrated in Scheme 1b for reaction with ZntBu2 which afforded NHC-complex 3 in a 76% yield, proving by analogy that this unprecedented direct C–H to C–Zn transformation accomplished by 1 is a genuine example of a synergic bimetallic-induced reaction.21 Interestingly, as mentioned above, Robinson recently reported a more conventional two-step metathetical methodology involving generation of an anionic dicarbene by C4-lithiation of IPr15 followed by transmetallation with the zinc alkyl ZnEt2 which led to the isolation of a zincate species structurally akin to 2.18
The substrate scope of this bimetallic approach was probed by studying the reactions of 1 with other unsaturated carbenes. NMR spectroscopic studies (1H and 13C) confirmed that the selective C4-zincation of alkyl-substituted NHC 1,3-di-tert-butylimidazole-2-ylidene (IBu) can be readily accomplished to afford mixed sodium–zinc species 2IBu. Isolable as a crystalline solid in 63% yield, 2IBu has a similar constitution to that described for 2 (see ESI for experimental details†). Reactions with 1,3-bis(1-adamantylimidazole-2-ylidene) (IAd) and 1,3-dimesitylimidazole-2-ylidene (IMes) were also studied. Although no crystalline products could be isolated despite several attempts, comparison of the 2H NMR spectra of the in situ CD3OD quenched crude reaction mixtures with the 1H NMR spectra of the free carbenes show that deuterium incorporation has taken place in the backbone of the imidazole rings (see ESI for details†), thus matching the metallation seen directly in 2 and 2IBu.
Retention of two tBu groups on Zn in 2 is particularly noteworthy, showing that in this case heteroleptic zincate 1 has acted as an amido-base. This is in sharp contrast with previous AMMZn examples of aromatic and heterocyclic molecules, where the reaction does not stop there but in a fast second step, the amine coproduct TMP(H) is deprotonated by the bimetallic intermediate to reform a Na–TMP–Zn bridge and to eliminate isobutane irreversibly (Scheme 2).5–9 Thus considering previous mechanistic studies showing that zincate 1 executes deprotonation by a combination of kinetic amide/thermodynamic alkyl basicity,9,22 2 can be envisaged as a novel kinetic intermediate of AMMZn. Surprisingly, no subsequent reaction with the released TMP(H) (step 2 in Scheme 2) was observed, even under forcing refluxing conditions. A contributing factor for this may be the remoteness of a suitable Lewis acidic site to the zincate anion present in 2 to which TMP(H) could precoordinate (a requisite for step 2 to take place), as indicated by the large Na–Zn separation [6.561(2) Å]. This is due to the multi-atom-span (CNC) construction of the metal–metal' bridge compared to the single-atom bridge (N) of TMP. Furthermore, the fact that Na is coordinatively saturated by bonding to the normal C2 position of the metallated carbene and three molecules of THF must greatly hinder any possible interaction with the sterically restricted and poorer Lewis base TMP(H). This finding has important mechanistic implications to this bimetallic metalating approach as it shows that by modulating the ligand coordination of the alkali-metal (in the case of 2 by forming a complex with the anionic NHC with Na via its normal C2 position), it is possible to suppress the second step of AMMZn, which as recently demonstrated, can greatly influence the final regioselectivity of the Zn–H exchange process.9b
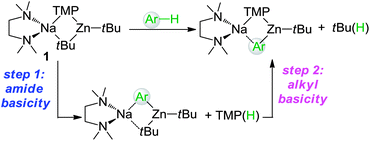 | ||
| Scheme 2 Two-step deprotonation process in AMMZn of arenes using TMP–zincate 1. | ||
Endeavouring to extend the AMMZn concept to NHC-complexes, we next subjected 3 to the bimetallic reagent 1 in hexane. Successful synergic C4-zincation of 3 afforded the novel solvent-separated ion pair zinczincate [Na(THF)6]+ [tBu2Zn:C{[N(2,6-iPr2C6H3)]2CHCZn(tBu2)}]− (4) in an isolated 30% yield23 (Scheme 1c). NMR spectroscopic analysis of 4 in d8-THF showed a singlet at 6.88 ppm for imidazole CH in the 1H NMR spectrum and a resonance at 160.8 ppm in the 13C NMR spectrum which can be assigned to the metallated C4 (akin to those found for 2, at 6.66 and 159.4 ppm respectively). In addition, the C2 resonance of the imidazole ring appears at 187.8 ppm in the 13C NMR spectrum, significantly upfield to that of 2 (at 201.4 ppm), consistent with the retention of the Zn–Ccarbene interaction.24
X-ray crystallographic studies established the molecular structure of 4 (Fig. 2) providing further confirmation that the direct C4 zincation of 3 has successfully been accomplished with the retention of the original Zn–Ccarbene bond. Exhibiting a solvent-separated ion pair structure, 4, contains an octahedral Na cation solvated by six THF molecules whereas its anionic counterion comprises an anionic IPr fragment, resulting from its deprotonation at the C4 of the imidazole ring which bridges two ZntBu2 units using its normal [i.e. C1] and abnormal [i.e. C2] positions. Inspecting the Zn–C distances in 4 shows that the Zn–C4 bond [Zn1–C2, 2.058(3) Å] is identical to that found for 2 and about 0.06 Å shorter than the Zn–C2 bond [Zn2–C1, 2.114(3) Å], which in turn compares well with that found in the NHC complex 3 [Zn2–C1, 2.118(5) Å]. As far as we can ascertain 4 represents the first example of metalation of a Zn–NHC complex. Mimicking the reaction with free IPr, bimetallic base 1 performs the zincation of 3 exclusively as an amido-TMP base, without observing further reaction of TMP(H) with any of the tert-butyl groups of the zincate. Since the C2 coordination site of the metallated carbene in 4 is apparently blocked by a ZntBu2 group, it could be expected that the regeneration of the Na–TMP–Zn bridge would be feasible furnishing a contacted ion-pair intermediate, similar to that observed for the AMMZn product of N-methylpyrrole by bimetallic base 1,20 where Na attains further stabilization by π-engaging with one of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C units of the heterocycle. However it should be noted that to form these π-interactions the alkali-metal requires to adopt a perpendicular disposition to the metallated ring, a mode of interaction sterically blocked here by the bulky Dipp substituents.
C units of the heterocycle. However it should be noted that to form these π-interactions the alkali-metal requires to adopt a perpendicular disposition to the metallated ring, a mode of interaction sterically blocked here by the bulky Dipp substituents.
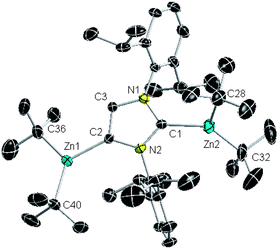 | ||
| Fig. 2 Structure of the anion present in 4 with 50% probability displacement ellipsoids. Hydrogen atoms and minor disorder in a tBu group omitted for clarity. | ||
Reaction of 3 with tBuLi: co-complexation vs. C4-metallation
To compare this mixed-metal approach with a more traditional lithium-mediated methodology, we then reacted NHC complex 3 with one molar equivalent of tBuLi in hexane (Scheme 1d). Deviating remarkably from the reactivity of 1, tBuLi fails to promote deprotonation of the coordinated carbene ligand instead forming homoleptic lithium zincate [IPrLiZntBu3] (5) (isolated in an 82% yield), which was analysed by multinuclear (1H, 13C and 7Li) NMR spectroscopy and X-ray crystallography (see ESI† and Fig. 3). Representing to the best of our knowledge the first example of an adduct between a neutral NHC and a zincate species, 5 has a contacted ion-pair structure, where the metals are connected by a double tBu bridge, with Li completing its coordination sphere by bonding to the carbene carbon of IPr while Zn binds to a terminal alkyl group. Interestingly, recent reports have shown that lithium zincates containing tert-butyl groups are chemoselective reagents utilised in several key organic transformations including for example Zn–halogen exchange of functionalised organic halides, SN2 reactions and ionic polymerizations of vinylamides.25 Nevertheless, despite spectroscopic and theoretical studies on the constitution of these mixed-metal species, their structures in the solid state have not yet been elucidated. Furthermore, these studies have shown that in THF solution LiZntBu3 readily redistributes to higher order Li2ZntBu4 and ZntBu225c suggesting that the coordination of NHC to the tri(alkyl) lithium zincate in 5 is highly stabilising, suppressing this decomposition pathway. It should also be noted that 1H NMR monitoring of solutions of 5 in deuterated benzene over 48 h did not show any evidence of disproportionation or deprotonation of coordinated NHC. This finding contrasts with the facile lithiation of free IPr using nBuLi as a base reported by Robinson,15 showing not only that the co-complexation reaction of both homometallic alkyls to yield a lithium zincate is favoured over the deprotonation process, but also that by becoming a constituent of this bimetallic species, there is a marked depreciation of the metallating power of tBuLi.26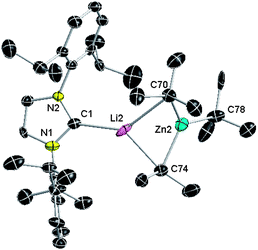 | ||
| Fig. 3 Molecular structure of 5 with 50% probability displacement ellipsoids. Hydrogen atoms omitted for clarity. | ||
DFT calculations
Theoretical calculations at the DFT level employing the B3LYP method and the 6-311G** basis set were used to model the metalation reactions of IPr and NHC complex 3 by synergic base 1 (see ESI for details†), showing that the formation of products 2 and 4 respectively along with the release of concomitant TMP(H) is energetically favoured (by −23.9 kcal mol−1 for 2A and −32.5 kcal mol−1 for 4A; with 2A and 4A being the modelled structures of products 2 and 4 respectively). The fact that these reactions are largely thermodynamically driven is particularly noteworthy when compared with previous theoretical studies by Uchiyama and Nobuto, which show that for the zincation of benzene using heteroleptic base 1, it is significantly more favoured, from a merely thermodynamic point of view, for 1 to act in an single step as an alkyl base with the overall loss of isobutane (Scheme 2) than just as a TMP-base.22 In fact, despite its kinetic preference, the amido basicity of 1 in the AMMZn of benzene was calculated to be slightly endothermic. This energy loss is however compensated for by the second step of the process, with the irreversible loss of gaseous isobutane (Scheme 2).Comparing the calculated geometrical parameters of the modelled structures 2A and 4A with those found experimentally from the X-ray crystallographic studies of 2 and 4, shows, in general, an excellent agreement (see Tables S2 and S3 in ESI†) although a slight underestimation is observed for the calculated length of the Zn–C4 (abnormal) interactions [2.127 Å (calc.) vs. 2.058(3) Å (exp) for 2; 2.135 Å (calc.) vs. 2.058(3) Å (exp) for 4].27 Natural bond orbital (NBO) analysis of 2A and 4A indicates that in both models the majority of the positive charge is carried primarily by the metals, showing significant dicationic character for the Zn atoms (calculated natural charges of Zn, +1.49 in 2A, and +1.50 and +1.49 in 4A).
Interestingly, calculations on the regioisomeric structure of 2A, where the positions of the {ZntBu2}and {Na(THF)3}+ fragments were exchanged, giving rise to Zn–C2 normal and Na–C4 abnormal coordination modes (2B in ESI†), showed that this model structure is only marginally less stable (by +3.5 kcal mol−1) than 2A. Notwithstanding, it should be noted that 1H and 13C NMR monitoring of solutions of 2 in deuterated THF solutions over 12 hours did not show any evidence of metal scrambling between the distinct C2/C4 coordination sites.
Fig. 4 shows the two highest occupied molecular orbitals calculated for model 2A which correspond to the Zn–C bonding orbitals at the quaternary carbon centers of the tBu groups and the C4 of the anionic NHC.27 This calculation contrasts with those reported for the related anionic lithium dicarbene [:C{[N(2,6-iPr2C6H3)]2CHCLi(THF)}]n prepared by Robinson,15 whose HOMO and HOMO-2 correspond to the two strongly polarised Li–C bonding orbitals at the C2 and C4 positions of the imidazole ring, suggesting that in mixed Na–Zn compound 2, the negative charge of the anionic NHC is primarily localised at its abnormal C4 position, which can attain extra-stabilisation by generation of a more covalent Zn–C bond. Contrastingly in the lithium polymer, C4 now binds to a much more electropositive Li centre, forming a significantly more polarised metal–carbon bond and facilitating the more effective delocalisation of its negative charge throughout the imidazole ring, hence its dicarbene character.
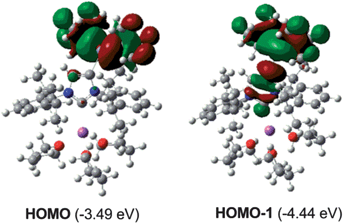 | ||
| Fig. 4 Calculated molecular orbitals HOMO and HOMO-1 of 2. | ||
Double transmetallation reaction to a Au(I) complex
Exploiting the excellent ability of organozinc reagents to undergo transmetallation reactions with transition metal complexes,1,2 we next decided to probe if sodium zincate 2 could act as a selective transfer agent of its anionic carbene fragment towards a transition metal complex. For this study, considering the isolobal analogy between {Au(L)}+ (L = neutral donor) and proton [H]+ cations,28 we chose Au(I) complex [ClAu(PPh3)] as a precursor. Thus the reaction of equimolar amounts of this complex with 2 furnished the unusual digold complex [ClAu:C{[N(2,6-iPr2C6H3)]2CHCAu(PPh3)}] (6) in a modest 26% yield29 which can be increased to 61% when two equivalents of [ClAu(PPh3)] are employed (Scheme 3, see ESI for experimental details and NMR spectroscopic characterization†).![Transmetallation reaction of 2 to [ClAu(PPh3)].](/image/article/2013/SC/c3sc52101j/c3sc52101j-s3.gif) | ||
| Scheme 3 Transmetallation reaction of 2 to [ClAu(PPh3)]. | ||
X-ray crystallographic studies of 6 confirmed that a double transmetallation reaction has taken place, where the anionic carbene {:C{[N(2,6-iPr2C6H3)]2CHC} links two Au(I) centers (Fig. 5), each of them in distinct chemical environments. Thus, while Au(1) occupies the position previously filled by ZntBu2 in 2, binding to the C4 (abnormal) position [i.e. C2] and triphenylphosphine, Au(2) coordinates to the classical C2 position of the anionic carbene [i.e. C1] and to Cl. The Au atoms are separated by 6.076(1) Å which precludes any possible aurophilic interaction.30 Examining the geometrical parameters of 6 reveals that while the Au2–C1 bond distance [1.975(5) Å] is similar to those in other neutral NHC–Au carbene complexes [e.g., IPrAuCl, 1.942(3) Å],31 Au1–C2 bond distance is considerably elongated [2.022(5) Å]. In fact this distance is closer to those reported for related [(aryl)Au(PPh3)] [aryl = Ph, 2.055 Å]32 derivatives, diverging significantly from the Au–C bond distance reported for the only example in the literature of a neutral unsaturated abnormal NHC gold complex isolated by Bertrand et al. [1.981 Å],16c which is consistent with the interpretation of {:C{[N(2,6-iPr2C6H3)]2CHC} in 6 as an anionic carbene ligand.33 There are no precedents in the literature for coordination of this type of ligand to two transition metal centres, with the closest analogy being a recently published mixed K–Mn species resulting from the reduction of a neutral Mn(II) NHC complex by KC8.34 Establishing a new methodology for accessing dinuclear organogold species under mild reaction conditions, the synthesis of 6 demonstrates the dual transmetallating ability of 2 which reacts differently with each equivalent of [ClAu(PPh3)]. Thus, one equivalent reacts with the zincate anion of 2 which selectively transfers its anionic carbene fragment in preference to its tBu groups to Au,35 with the subsequent precipitation of NaCl and elimination of ZntBu2 (Scheme 3). This creates a coordination vacancy in the C2 position of the anionic carbene (originally bound to Na) which can in turn react with a second equivalent of [ClAu(PPh3)], ejecting the neutral phosphine ligand. Remarkably, even when equimolar amounts of [ClAu(PPh3)] and 2 are employed, digold species 6 is still obtained although the yield of the reaction is greatly diminished (to 26%, maximum possible 50%). Thus, the formation of a putative mixed gold–zinc species, resulting from the coordination of the concomitantly formed ZntBu2 to the normal C2 position of the anionic carbene is not observed. The double transmetallation observed here contrasts with recent studies published by Tamm et al. on the reactivity of [ClAu(PPh3)] with an anionic lithium carbene containing a borate moiety in its imidazole backbone. This enabled the isolation of a neutral mononuclear gold species, resulting from the single exchange of the C2 position of Li (which is eliminated as LiCl) and the {Au(PPh3)}+ fragment, keeping the borate part of the NHC intact.36
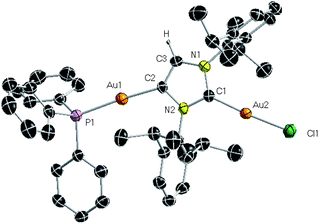 | ||
| Fig. 5 Molecular structure of 6 with 50% probability displacement ellipsoids. Hydrogen atoms except on the backbone of the imidazole ring omitted for clarity. | ||
Interestingly, reflecting the more kinetically retarded character of the Zn–C bonds in this in situ generated neutral organozinc species, no further transmetallation reaction was observed between 6 (which still possesses a potentially reactive Au–Cl bond) and ZntBu2, even when longer reaction times (up to 24 hours) were allowed. It should also be noted that the ancillary PPh3 ligand in the Au(I) precursor also plays a critical stabilising role in this reaction as the treatment of 2 with one equivalent of related [ClAu(SMe2)], containing a more labile neutral ligand, led to the formation of intractable black solutions.
Conclusions
In summary, by merging two current evolving areas in synthesis, namely cooperative bimetallic reagents and NHCs, we have isolated the first intermediates of AMMZn of a free NHC and Zn–NHC complex which provide important mechanistic insights into how (TMP)-based alkali-metal zincates operate as well as a new synthetic tool for the functionalisation of the unsaturated backbone of NHC ligands. With the isolation of homoleptic IPr·LiZntBu3 we show for the first time the ability of the commodity ligands to provide stabilization to sensitive zincate species, suppressing disproportionation processes. The potential that sodium zincate 2 can offer as a transfer agent of its anionic NHC ligand to transition metal complexes has been realized by the isolation of the novel digold species 6. Considering the broad catalytic activity exhibited by Au(I)–NHC complexes37 as well as recent reports that have highlighted the importance of digold species and their possible role in catalytic transformations,38 the synthesis of 6 where each Au centre has a distinct chemical environment may lead to new advances in this topical field. Furthermore, the investigation of transmetallation reactions of sodium zincate 2 with other transition metal complexes may break new ground on the synthesis of novel complexes containing anionic NHC ligands.Experimental section
Full experimental and computational details and characterization of compounds 2–6 are included in the ESI.†Acknowledgements
We thank the EPSRC, the Royal Society, the European Research Council (ERC), and the Carnegie Trust for the Universities of Scotland for their generous sponsorship of this research. J.G.-A. thanks the MICINN and the European Social Fund for the award of a “Ramón y Cajal” contract. We also thank Professor Mulvey for his insightful comments and Denise Gilmour for her assistance in the CHN analysis of highly air and moisture sensitive compounds 2–5.References
- P. Knochel and P. Jones, in Organozinc Reagents: A Practical Approach, ed. L. H. Harwood and C. J. Moody, Oxford University Press, Oxford, 1999 Search PubMed.
- The chemistry of organozinc compounds, ed. Z. Rappoport and I. Marek, Patai Series, Wiley, Chichester, UK, 2006 Search PubMed.
- For recent reviews in organozincate chemistry and their applications in synthesis see: (a) R. E. Mulvey, Organometallics, 2006, 25, 1060 CrossRef CAS; (b) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802 CrossRef CAS PubMed; (c) R. E. Mulvey, Acc. Chem. Res., 2009, 42, 743 CrossRef CAS PubMed; (d) D. J. Linton, P. Schooler and A. E. H. Wheatley, Coord. Chem. Rev., 2001, 223, 53 CrossRef CAS; (e) M. Westerhausen, Dalton Trans., 2006, 4755 RSC; (f) B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9794 CrossRef CAS PubMed; (g) R. E. Mulvey, Dalton Trans., 2013, 42, 6676 RSC.
- J. Clayden, Organolithiums: Selectivity for Synthesis, Pergamon, Elsevier Science Ltd., Oxford, UK, 2002 Search PubMed.
- P. C. Andrikopoulos, D. R. Armstrong, H. R. L. Barley, W. Clegg, S. H. Dale, E. Hevia, G. W. Honeyman, A. R. Kennedy and R. E. Mulvey, J. Am. Chem. Soc., 2005, 127, 6184 CrossRef CAS PubMed.
- D. R. Armstrong, W. Clegg, S. H. Dale, D. V. Graham, E. Hevia, L. M. Hogg, G. W. Honeyman, A. R. Kennedy and R. E. Mulvey, Chem. Commun., 2007, 598 RSC.
- W. Clegg, S. H. Dale, E. Hevia, L. M. Hogg, G. W. Honeyman, R. E. Mulvey and C. T. O'Hara, Angew. Chem., Int. Ed., 2006, 45, 6548 CrossRef CAS PubMed.
- For selected references see: (a) D. R. Armstrong, W. Clegg, S. H. Dale, E. Hevia, L. M. Hogg, G. W. Honeyman and R. E. Mulvey, Angew. Chem., Int. Ed., 2006, 45, 3775 CrossRef CAS PubMed; (b) W. Clegg, S. H. Dale, A. M. Drummond, E. Hevia, G. W. Honeyman and R. E. Mulvey, J. Am. Chem. Soc., 2006, 128, 7434 CrossRef CAS PubMed; (c) D. R. Armstrong, J. Garcia-Alvarez, D. V. Graham, G. W. Honeyman, E. Hevia, A. R. Kennedy and R. E. Mulvey, Chem.–Eur. J., 2009, 15, 3800 CrossRef CAS PubMed.
- (a) W. Clegg, B. Conway, E. Hevia, M. D. McCall, L. Russo and R. E. Mulvey, J. Am. Chem. Soc., 2009, 131, 2375 CrossRef CAS PubMed; (b) D. R. Armstrong, V. L. Blair, W. Clegg, S. H. Dale, J. Garcia-Alvarez, G. W. Honeyman, E. Hevia, R. E. Mulvey and L. Russo, J. Am. Chem. Soc., 2010, 132, 9480 CrossRef CAS PubMed.
- A. J. Arduengo, III, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef.
- For selected references see: (a) D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 2000, 100, 39 CrossRef CAS PubMed; (b) A. S. K. Hashmi, C. Lothschütz, C. Böhling, T. Hengst, C. Hubbert and F. Rominger, Adv. Synth. Catal., 2010, 352, 3001 CrossRef CAS; (c) A. S. K. Hashmi, A. M. Schuster and F. Rominger, Angew. Chem., Int. Ed., 2009, 48, 8247 CrossRef CAS PubMed; (d) G. Guisado-Barrios, J. Bouffard, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 4759 CrossRef CAS PubMed.
- (a) S. P. Nolan, N-Heterocyclic Carbenes in Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed; (b) S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef CAS PubMed; (c) N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis, ed. C. S. J. Cazin, Springer, London, 2011 Search PubMed.
- For selected examples see: (a) Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer, III, P. v. R. Schleyer and G. H. Robinson, Science, 2008, 321, 1069 CrossRef CAS PubMed; (b) R. S. Ghadwal, H. W. Roesky, S. Merkel, J. Henn and D. Stalke, Angew. Chem., Int. Ed., 2009, 48, 5683 CrossRef CAS PubMed; (c) J. D. Masuda, W. W. Schoeller, B. Donnadieu and G. Bertrand, J. Am. Chem. Soc., 2007, 129, 14180 CrossRef CAS PubMed; (d) O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369 CrossRef CAS PubMed; (e) Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer, III, P. v. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2008, 130, 14970 CrossRef CAS PubMed.
- (a) D. Mendoza-Espinosa, B. Donnadieu and G. Bertrand, J. Am. Chem. Soc., 2010, 132, 7264 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, Y. Abraham, P. Wei, H. F. Schaefer, III, P. v. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2010, 132, 14370 CrossRef CAS PubMed.
- NHCs which coordinate to a metal center through a backbone C4 or C5 carbon have been branded as abnormal carbenes, (aNHCs): (a) P. L. Arnold and S. Pearson, Coord. Chem. Rev., 2007, 251, 596 CrossRef CAS PubMed; (b) O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445 CrossRef CAS PubMed; (c) E. Aldeco-Perez, A. J. Rosenthal, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Science, 2009, 326, 556 CrossRef CAS PubMed; (d) R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755 CrossRef CAS PubMed.
- Previous to this work Arnold et al. have described the isolation of a bimetallic potassium/yttrium anionic N-heterocyclic dicarbene complex resulting from the reduction of an yttrium NHC complex with potassium naphthalenide which is formally a deprotonation product. P. L. Arnold and S. T. Liddle, Organometallics, 2006, 25, 1485 CrossRef CAS.
- Y. Wang, Y. Xie, M. Y. Abraham, R. G. Gilliard, Jr, P. Wei, C. F. Campana, H. F. Schaefer, III, P. v. R. Schleyer and G. H. Robinson, Angew. Chem., Int. Ed., 2012, 51, 10173 CrossRef CAS PubMed.
- Initially reactions were carried out in neat hexane, as bimetallic base 1 degrades rapidly in THF solutions. On the addition of IPr or complex 3 insoluble white precipitates were formed instantaneously, suggesting that the metallation reactions have taken place. THF was then introduced to allow these solids to dissolve and facilitate the crystallisation of 2 and 4 respectively.
- B. Conway, E. Hevia, A. R. Kennedy and R. E. Mulvey, Chem. Commun., 2007, 2864 RSC.
- Highlighting the poor kinetic basicity of Zn–C bonds in simple organozinc reagents, it should be noted that no metalation of IPr was observed even when a 3-fold molar excess ZntBu2 was employed.
- D. Nobuto and M. Uchiyama, J. Org. Chem., 2008, 73, 1117 CrossRef CAS PubMed.
- NMR analysis of the filtrate showed that 4 is the only organometallic species present in solution suggesting that its formation is quantitative.
- For selected examples of NHC–zinc complexes see: (a) A. J. Arduengo, III, H. V. R. Dias, F. Davidson and R. L. Harlow, J. Organomet. Chem., 1993, 462, 13 CrossRef; (b) P. L. Arnold, I. J. Casey, Z. R. Turner, R. Bellabarba and R. B. Tooze, Dalton Trans., 2009, 7236 RSC; (c) B. Bantu, G. Manohar Pawar, K. Wurst, U. Decker, A. M. Schmidt and M. R. Buchmeiser, Eur. J. Inorg. Chem., 2009, 1970 CrossRef CAS.
- (a) M. Kobayashi, Y. Matsumoto, M. Uchiyama and T. Ohwada, Macromolecules, 2004, 37, 4339 CrossRef CAS; (b) M. Uchiyama, T. Furuyama, M. Kobayashi, Y. Matsumoto and K. Tanaka, J. Am. Chem. Soc., 2006, 128, 8404 CrossRef CAS PubMed; (c) T. Furuyama, M. Yonehara, S. Arimoto, M. Kobayashi, Y. Matsumoto and M. Uchiyama, Chem.–Eur. J., 2008, 14, 10348 CrossRef CAS PubMed.
- For a related example on the attenuation of deprotonating power of a sodium alkyl reagent towards toluene by forming a sodium magnesiate species see: S. E. Baillie, W. Clegg, P. Garcia-Alvarez, E. Hevia, A. R. Kennedy, J. Klett and L. Russo, Chem. Commun., 2011, 47, 388 RSC.
- DFT calculations on the constitution of the HOMO and the LUMO of compounds 2A and 4A can be found in the ESI.†.
- For an insightful review on gold chemistry and the isolability concept see: H. G. Raubnheimer and H. Schmidbaur, Organometallics, 2012, 31, 2507 CrossRef.
- Note that employing this stoichiometry, the maximum possible yield for 6, with respect to gold precursor [ClAu(PPh3)], is 50%.
- K. R. Flower, A. T. McGown, P. J. Miles, R. G. Pritchard and J. E. Warren, Dalton Trans., 2010, 39, 3509 RSC.
- P. de Frémont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 2411 CrossRef.
- A. S. K. Hashmi, T. D. Ramamurthi and F. Rominger, J. Organomet. Chem., 2009, 694, 592 CrossRef CAS PubMed.
- For other recent examples of saturated abnormal NHC–gold complexes see: A. S. K. Hashmi, D. Riedel, M. Rudolph, F. Rominger and T. Oeser, Chem.–Eur. J., 2012, 18, 3827 CrossRef CAS PubMed . The Au–carbene bond lengths in these compounds range from 1.957(6) to 1.977(3) Å.
- R. A. Musgrave, R. S. P. Turbevill, M. Irwin and J. M. Goicoechea, Angew. Chem., Int. Ed., 2012, 51, 10832 CrossRef CAS PubMed.
- Although 6 was isolated in a 61% crystalline yield, NMR analysis of the filtrate of the reaction showed that it is the only organometallic species present in solution. No signals which could be assigned to tBu groups could be observed neither in the 1H or 13C NMR spectra. This can be attributed to the high volatility of ZntBu2 (one of the side products generated in this exchange reaction) which can then be easily removed under vacuum.
- S. Kronig, E. Theuergarten, C. G. Daniliuc, P. G. Jones and M. Tamm, Angew. Chem., Int. Ed., 2012, 51, 3240 CrossRef CAS PubMed.
- For selected references see: (a) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (b) E. Jimenez-Nunez and A. M. Echevarren, Chem. Commun., 2007, 333 RSC; (c) M. Rudolph and A. S. K. Hashmi, Chem. Commun., 2011, 47, 6536 RSC; (d) M. Rudolph and A. S. K. Hashmi, Chem. Soc. Rev., 2012, 41, 2448 RSC.
- (a) A. C. Gomez-Suarez and S. P. Nolan, Angew. Chem., Int. Ed., 2012, 51, 8156 CrossRef CAS PubMed and references therein; (b) A. S. K. Hashmi, T. Lauterbach, P. Nösel, M. H. Vilhelmsen, M. Rudolph and F. Rominger, Chem.–Eur. J., 2013, 19, 1058 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CIF files giving crystallographic results, experimental details and copies of the NMR spectra. CCDC 923037–923041. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3sc52101j |
| This journal is © The Royal Society of Chemistry 2013 |
