 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of cell-impermeable coelenterazine derivatives†
Eric Lindberga, Shin Mizukamiab, Keiji Ibatacd, Takashi Fukanod, Atsushi Miyawakide and Kazuya Kikuchi*ab
aGraduate School of Engineering, Osaka University, Osaka 565-0871, Japan. E-mail: kkikuchi@mls.eng.osaka-u.ac.jp; Fax: +81-6-6879-7924; Tel: +81-6-6879-7875
bImmunology Frontier Research Center, Osaka University, Osaka 565-0871, Japan
cDepartment of Physiology, School of Medicine, Keio University, Tokyo, Japan
dRIKEN Brain Science Institute, Laboratory for Cell Function Dynamics, Saitama 351-0198, Japan
eLife, Function and Dynamics, Exploratory Research for Advanced Technology, Japan Science and Technology Agency, Saitama, Japan
First published on 3rd September 2013
Abstract
We describe the development of the first cell-membrane impermeable coelenterazine derivative (CoelPhos). CoelPhos was constructed by the alkylation of coelenterazine with a linker containing a terminal anionic phosphonate moiety. The bioluminescence activity of CoelPhos with Gaussia luciferase (GLuc) showed a significantly higher activity in comparison with Renilla luciferase. In imaging studies with living cells, outer membrane bound GLuc was clearly imaged with CoelPhos. On the other hand no signal could be detected with intracellularly localized GLuc, demonstrating the impermeability of this novel coelenterate substrate derivative. CoelPhos has potential utility as a new bioluminogenic tool for the monitoring of dynamic fusion events at the cell–surface interface.
1 Introduction
Bioluminescence imaging (BLI) is a valuable technique that is readily used in the life sciences. Bioluminescence is a natural phenomenon that involves the conversion of chemical energy into the emission of visible light. A luciferase enzyme catalyzes the chemical reaction between a luciferin substrate and molecular oxygen, generating a high energy species which, upon decaying, results in the emission of light. Firefly and Renilla luciferases are the most commonly used luciferases, with D-luciferin and coelenterazine as their respective bioluminogenic substrates. Firefly luciferase (FLuc) is usually the preferred choice due to its more favorable emission spectrum (560 nm). It is not suitable though, for the monitoring of extracellular events due to the requirement of intracellular co-factors such as ATP, which can activate purinergic ion-channel receptors. Coelenterate luciferases on the other hand do not require extra co-factors besides molecular oxygen. Gaussia luciferase (GLuc) is the brightest among all reported luciferases and displays a flash-type luminescence profile.1 These luciferases have mostly been used as reporters to monitor dynamic changes in gene expression and transcription.2The monitoring of single exocytotic events in living cells has mostly employed methods such as total internal reflection fluorescence (TIRF), and two-photon laser scanning microscopy.3–6 However, these methods are confined to monitoring a limited section of the cells and the use of fluorogenic dyes for accurate localization can be difficult due to diffusion. In addition, fluorescence methods require continuous irradiation, causing cell damage and photobleaching. Moreover due to their temporal resolution properties, confocal and two-photon laser scanning microscopies are more suitable when monitoring the membrane fusion of vesicles with slower kinetics.7
BLI can offer distinct advantages over fluorescence imaging in the monitoring of protein secretion and other exocytotic events in living cells. The visualization of luciferase secretion in living mammalian cells was first realized with Cypridina luciferase (CLuc) in CHO cells,8 and in live mouse embryos to monitor the transcriptional activation of genes.9 CLuc was also utilized for imaging neurotransmitter release.10 Recently secretion of the brighter GLuc in PC12D cells was monitored in real-time.11 However, in general BLI has suffered from poor resolution due to low luminescence intensity. Using an electron multiplying charge coupled device (EM-CCD) camera, video-rate BLI was employed in studying the secretory dynamics of MMP-2 with GLuc at much improved resolutions.12 In another study, the same group also demonstrated that video-rate BLI could be used for the quantitative analysis of insulin oscillations in pancreatic MIN6 β cells, which could have potential drug-screening applications.13
Recently, in an effort to monitor exocytosis in synaptic boutons via BLI, a mutant GLuc with enhanced luminescence output was fused to the part of a vesicle-associated membrane protein (VAMP) located within the interior of the synaptic vesicle (unpublished data). Hence, when the vesicle fuses with the cell membrane, undergoing exocytosis, the luciferase would react with its substrate coelenterazine, generating a bioluminescent response. However, a very poor signal-to-noise ratio was observed which was attributed to the high cell-membrane permeability of coelenterazine, i.e. coelenterazine could diffuse across the synaptic and vesicle membranes and react with the luciferase before the exocytotic event, giving rise to high background luminescence (Fig. 1a). Miesenbock et al. also observed a similar issue with Cypridina luciferin when imaging patterns of synaptic activity in hippocampal neuronal cells.10 Therefore a modified coelenterazine substrate with decreased cell-membrane permeability is desired (Fig. 1b and c). However modifying the bioluminogenic substrate without having a negative impact on the bioluminescence activity is very difficult. This is especially true in the case of GLuc, which has very high substrate specificity. Hence, even the smallest modification of the coelenterazine substrate will result in a significant drop in bioluminescence activity.14–16 Therefore it is not surprising that only one coelenterazine derivative (s-CTZ) with improved bioluminescence activity with GLuc has been reported.17 The compound structure has yet to be published, however, the published data strongly implies that s-CTZ is highly cell-permeable. Hence we sought to design and synthesize a cell-membrane impermeable coelenterazine derivative with retained bioluminescence activity.
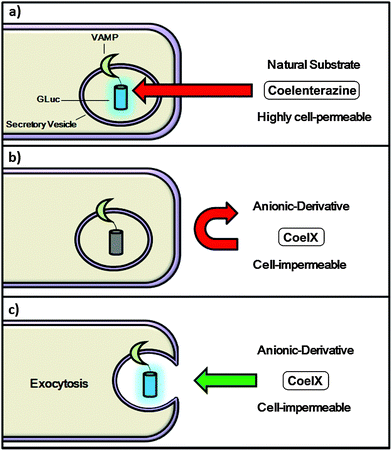 | ||
| Fig. 1 Monitoring membrane fusion events via BLI; concept (a) coelenterazine readily penetrates the cell-membrane, giving rise to background noise prior to fusion taking place; (b) coelenterazine derivative CoelX has a negative charge, making it highly cell-impermeable and unable to react with GLuc; (c) following fusion of the secretory vesicle with the cell-membrane CoelX can react with GLuc, generating a bioluminescence signal. | ||
2 Results and discussion
2.1 Design and synthesis of 2-BnO-TEG-CTZ and 6-BnO-TEG-CTZ
When conceiving of a proper rational substrate design strategy, it is very useful to perform docking simulations of substrate derivatives before undertaking actual experimental work. Urano et al. constructed near-infrared-emitting firefly luciferins by initially performing docking simulations with luciferase from Photinus pyralis.18 However, as there is no existing X-ray crystal structure of Gaussia luciferase (GLuc), and a lack of homology with other coelenterate luciferases, for which crystal-structures are available, the rational design and modification of coelenterazine remains a difficult task in terms of being able to predict the effect of any modification of the coelenterazine substrate on bioluminescence activity. Modification of the coelenterazine substrate has mostly been focused on increasing light output, red-shifting the emission spectrum of coelenterazine and increasing the stability of coelenterazine to decrease autoluminescence background noise.19–24 As mentioned above, coelenterate luciferases share very little homology and hence any modification of coelenterazine that might have an advantageous impact on the luminescence output of one luciferase, might have the reverse effect on another. This is especially true for GLuc, which has been shown to have an extremely narrow substrate specificity.14–16 In order to construct a cell-impermeable derivative of coelenterazine, we decided to attach a flexible linker with a terminal anionic phosphonate group. Initially we synthesized two coelenterazine derivatives with a highly flexible polyethylene glycol (PEG) linker with terminal benzyl-protecting groups installed at the 2 and 6 positions of coelenterazine (Fig. 2) to investigate the effect on the bioluminescence activity of GLuc.We prepared 11 from tetraethylene glycol (TEG) by mono-benzylation with benzyl bromide, followed by bromination to 12via the Apple reaction (Scheme S2†). Synthetic intermediates 6 and 10 were synthesized as previously described (Scheme S1†).25,2613 was synthesized via standard alkylation of coelenteramine 6 with 12. Following acid-catalyzed condensation reaction of 13 and the α-ketoacetal 9 under reflux the desired compound 6-BnO-TEG-CTZ (2) was synthesized (Fig. 2). Synthesis of 2-BnO-TEG-CTZ (3) was accomplished by initial alkylation of deprotected α-ketoacetal 10 with the TEG linker 12 to give 14. Acid-catalyzed condensation reaction between 6 and 14 under reflux generated the desired product, 2-BnO-TEG-CTZ (3).
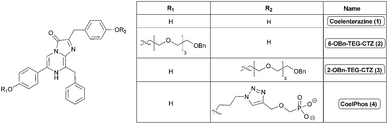 | ||
| Fig. 2 Structures of native coelenterazine and synthesized derivatives. | ||
Next the luminescent properties of 2- and 6-BnO-TEG-CTZ were evaluated with GLuc in comparison with native coelenterazine. Both substrates 2-BnO-TEG-CTZ and 6-BnO-TEG-CTZ showed more than 200- and 950-fold reduction in total bioluminescence activity, respectively (Table 1). Interestingly, Urano et al. observed similarly poor bioluminescence activity when attaching flexible PEG linkers to aminoluciferin.18 Installing rigid alkyl linkers also resulted in a low bioluminescence output. The poor observed activity was attributed to that these linkers may be linear and adopt conformations that hinder access of the luciferin substrate to the active site of the luciferase enzyme. Hence, it was concluded that long PEG linkers conjugated near the luminophore are unsuitable. This conclusion also seems to be valid in the case of installing PEG linkers on coelenterazine as well.
2.2 Design and synthesis of CoelPhos
Due to the detrimental effect on bioluminescence activity of PEG-modified coelenterazines we decided to change to more rigid alkyl linkers. Although our initial results suggested that both 2- and 6-positions of coelenterazine were sensitive to modification, we decided to focus on modification of the 2-position. We synthesized a phosphonate alkyne, dimethyl(prop-2-ynyloxy)methylphosphonate 16 in two steps (Scheme S3†). Alkylation of 10 with 1,3-dibromopropane resulted in the formation of 17, which was converted into the propyl azide 18. Copper(I)-catalyzed azide-alkyne Huisgen cycloaddition27 gave the desired 1,2,3-triazole 19. In the final step, acid-catalyzed condensation reaction of 6 and 19 was conducted, generating the desired product CoelPhos (Fig. 2) (see ESI† for further description and details).Next, we evaluated the luminescent properties of CoelPhos with GLuc (Table 1) and Renilla luciferase (RLuc) in comparison with coelenterazine (Table S1†). CoelPhos yielded a much stronger luminescence with GLuc than RLuc and also a significant improvement in bioluminescence activity compared with both 2- and 6-BnO-TEG-CTZ, although CoelPhos still showed about a 30-fold reduction in luminescence intensity in comparison with coelenterazine. With the exception of s-CTZ,17 the highest reported bioluminescence activities of any coelenterazine derivative with GLuc are those of MeO-CTZ (methylation of the 2-hydroxy group) (13.6%) and 3iso-CTZ (replacement of 2-hydroxy group with 3-isopropylbenzene), (14.3%).16
2.3 Bioluminescence imaging with CoelPhos
Recently GLuc mutants with improved luminescence output and stable luminescence profiles have been reported.28,29 We utilized a recently developed mutant GLuc (GLucM23) with roughly 10-fold improvement in luminescence intensity in living cells, and a glow-type kinetic profile (see ESI†). We evaluated CoelPhos by measuring bioluminescence activity with a charge-coupled device (CCD) camera in HeLa cells expressing outer-membrane localized wild-type GLuc or GLucM23 (GLucM23Mem) to determine if the improved mutant GLuc had a negative or positive impact on the luminescence intensity of CoelPhos (Fig. 3). We determined the relative intensities of coelenterazine and CoelPhos by comparing the relative mKO fluorescence and luminescence intensities of 5 cells (Fig. 4). CoelPhos showed similar relative bioluminescence activity to the mutant GLucM23 in comparison with the wild-type GLuc relative to coelenterazine (∼3–4%). Hence the GLuc mutant did not affect the bioluminescence activity of CoelPhos relative to coelenterazine. The contrast observed in HeLa cells expressing outer membrane bound GLuc was very poor in comparison with GLucM23. This is related to the flash-type luminescence of GLuc, together with the luminescence sensitivity of our imaging system. The stable glow-type luminescence of GLucM23 and its higher luminescence output makes it more suitable for BLI in living cells. This was especially true for our derivative CoelPhos which could be imaged with much improved signal-to-noise ratio with GLucM23Mem (Fig. 3k and l) over GLucMem (Fig. 3h and i).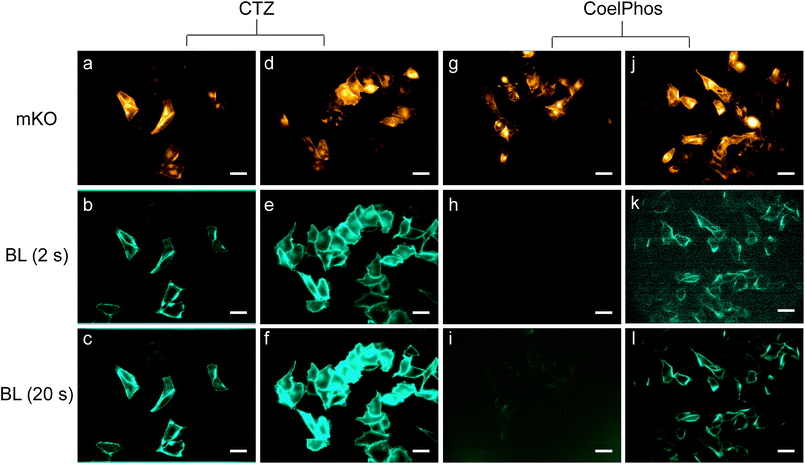 | ||
| Fig. 3 Bioluminescence imaging with coelenterazine (CTZ) and CoelPhos in HeLa cells expressing outer-membrane bound GLuc or GLucM23. (a, d, g and j) Fluorescence images of mKO (λex = 548 nm/λem = 559 nm; 200 ms) before addition of bioluminogenic substrate. CTZ with GLucMem, exposure time: 2 s (b) and 20 s (c); CTZ with GLucM23MeM, exposure time: 2 s (e) and 20 s (f); CoelPhos with GLucMem, exposure time: 2 s (h) and 20 s (i); CoelPhos with GLucM23Mem, exposure time: 2 s (k) and 20 s (l). Concentration of CTZ and CoelPhos: 22.7 μM. Objective lens: 60×. Scale bar: 40 μm. | ||
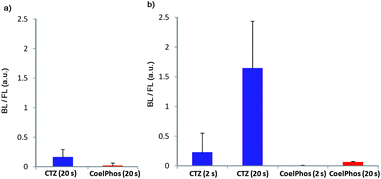 | ||
| Fig. 4 Relative activity of CoelPhos with GLuc and GLucM23 versus coelenterazine (CTZ) in HeLa cells. (a) CTZ and CoelPhos with GLuc; (b) CTZ and CoelPhos with GLucM23 (n = 5 cells); signal was quantified using ImageJ (2 or 20 s) = exposure time. | ||
Finally, we evaluated the relative cell-membrane permeability of our probe CoelPhos in comparison with coelenterazine. We transfected HeLa cells with an endoplasmic reticulum (ER)-localizing GLuc construct (GLucER). The construct also contained Venus fluorescent protein as a control for expression levels as well as confirmation of localization. We reasoned that whilst coelenterazine would give a strong signal due to its high cell-permeability, CoelPhos would not give any bioluminescence signal due to its inability to cross the cell-membrane (Fig. 5). When coelenterazine was added, strong bioluminescent signals were observed in HeLa cells expressing GLucER. On the other hand, when CoelPhos was added, no signal could be detected, even after extending the exposure time to 100 s (20-fold) (Fig. 6). The observed data seem to suggest that attachment of a terminal phosphonate moiety is enough to significantly decrease cell-membrane permeability of the coelenterate substrate.
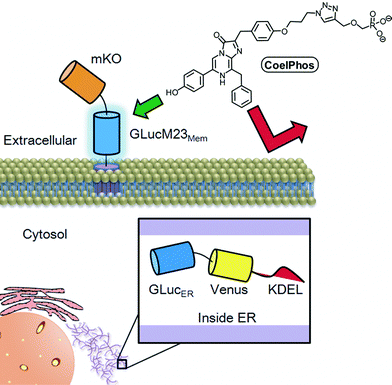 | ||
| Fig. 5 Illustration of the cell-permeability of CoelPhos. Because of the attached anionic phosphonate group CoelPhos does not penetrate the cell-membrane as readily as native coelenterazine. Therefore a bioluminescent signal will be observed with outer-membrane bound GLucM23Mem but not with intracellularly localized GLucER. | ||
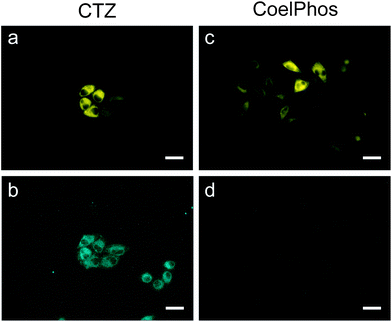 | ||
| Fig. 6 Fluorescence and bioluminescence images of HeLa cells expressing GLucER with coelenterazine (CTZ) and CoelPhos. (a) Venus FL; (b) CTZ (22.7 μM); exposure time: 5 s; (c) Venus FL; (d) CoelPhos (22.7 μM); exposure time: 100 s. Objective lens: 60×. Scale bar: 40 μm. | ||
It has been shown that GLuc produces a 1000-fold higher BL signal than RLuc or FLuc in mammalian cells.1,30 GLucM23 has roughly 10 times higher BL intensity over wild-type GLuc in mammalian cells, suggesting that this novel mutant GLuc could have a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold higher BL signal over RLuc and FLuc in mammalian cells. Although there was an over 30-fold decrease in BL activity of CoelPhos with GLuc and GLucM23 in comparison with native coelenterazine, it seems reasonable to assume that CoelPhos together with GLucM23 would show a stronger BL intensity over RLuc with coelenterazine. Further investigation is required however, in order to be able to make a direct comparison.
000-fold higher BL signal over RLuc and FLuc in mammalian cells. Although there was an over 30-fold decrease in BL activity of CoelPhos with GLuc and GLucM23 in comparison with native coelenterazine, it seems reasonable to assume that CoelPhos together with GLucM23 would show a stronger BL intensity over RLuc with coelenterazine. Further investigation is required however, in order to be able to make a direct comparison.
Not only does the substrate specificity of GLuc differ from other coelenterate luciferases such as Renilla and Oplophorus luciferase but GLuc has been shown to contain two catalytic domains; both with similarly narrow substrate specificities.30 Significant differences are also observed in the kinetic properties. Unlike other marine luciferases (RLuc, CLuc) which respond to their respective coelenterate luciferin concentration in a linear non-cooperative manner, it was recently shown that GLuc operates in a cooperative manner, possibly via an allosteric mechanism.31
Previous studies utilizing bioluminescence imaging for monitoring of real-time protein secretion showed the importance of the type of camera used. Exocytotic events of native GLuc in CHO-K1 cells were visualized in real time with a time resolution of 10 s. However, the resolution of the luminescence spots was low, which made it difficult to identify single exocytotic spots of luminescence due to the low luminescence sensitivity of the camera used.12 In more recent studies the same group utilized a more powerful EM-CCD which resulted in a 60-fold increase in sensitivity, which allowed the real-time monitoring of localization and dynamics of proteins on the surfaces of cells with millisecond temporal resolution.13,14 Notwithstanding the fact that the CCD camera employed in our imaging studies was inferior to cameras utilized in the above references in terms of quantum efficiency and signal-to-noise ratio, we were able to visualize CoelPhos with GLucM23Mem at a sampling rate of 2 s. Hence it is plausible to venture that utilization of EM-CCD cameras with improved sensitivity will allow bioluminescence imaging of CoelPhos with much improved temporal resolution and luminescence sensitivity.
3 Conclusions
In conclusion, we have developed a cell-membrane impermeable coelenterazine derivative, CoelPhos, as a potential tool for monitoring membrane fusion events in living cells. The derivative was constructed by alkylating the phenolic hydroxy group at the 2-position of coelenterazine with an alkyl linker containing a terminal phosphonate group. While displaying a 30-fold lower activity with GLuc compared with native coelenterazine, CoelPhos showed a high specificity for GLuc over RLuc with a 30-fold higher activity. By utilizing a new mutant GLuc, GLucM23, we were able to image membrane-localized GLucM23 despite the lower luminescence intensity of CoelPhos. We demonstrated that CoelPhos has decreased cell-membrane permeability in comparison with native coelenterazine. Our probe, CoelPhos, has the potential to be used as a cell-impermeable bioluminescent tool for the monitoring of exocytotic events.Acknowledgements
This work was supported in part by the Cabinet Office, Government of Japan and through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) from JSPS, by the Grant-in-Aid for Scientific Research from MEXT of Japan (nos 20675004, 22108519, 25620133, 25242072, and 24108724 for K.K.; 24685028, 24115513, and 24651259 for S.M.; 23700387 for K.I.,), by the CREST from JST, by the Grant-in-Aid from MHLW of Japan, by the Naito Foundation, by the Takeda Science Foundation, by the Magnetic Health Science foundation, and by the Asahi Glass Foundation. The authors are also grateful for a Japanese government scholarship (Monbukagakusho) for E.L. to perform his PhD thesis work in the international frontier biotechnology program. We would like to thank Dr Yutaro Kumagai and Prof. Shizuo Akira for providing us with the plasmid for expressing Renilla luciferase.Notes and references
- B. A. Tannous, D.-E. Kim, J. L. Fernandez, R. Weissleder and X. O. Breakefield, Mol. Ther., 2005, 11, 435–443 CrossRef CAS PubMed.
- T. Ozawa, H. Yoshimura and S. B. Kim, Anal. Chem., 2013, 85, 590–609 CrossRef CAS PubMed.
- I. Akopova, S. Tatur, M. Grygorczyk, R. Luchowski, I. Gryczynski, J. Boredjo and R. Grygorczyk, Purinergic Signalling, 2012, 8, 59–70 CrossRef CAS PubMed.
- J. G. Burchfield, J. A. Lopez, K. Mele, P. Vallotton and W. E. Hughes, Traffic, 2010, 11, 429–439 CrossRef CAS PubMed.
- N. Takahashi and H. Kasai, Endocr. J., 2007, 54, 337–346 CrossRef CAS PubMed.
- A. Oshima, T. Kojima, K. Dejima, Y. Hisa, H. Kasai and T. Nemoto, Cell Calcium, 2005, 37, 349–357 CrossRef CAS PubMed.
- M. Matsuzaki, G. C. R. Ellis-Davies, T. Nemoto, Y. Miyashita, M. Iino and H. Kasai, Nat. Neurosci., 2001, 4, 1086–1092 CrossRef CAS PubMed.
- S. Inouye, Y. Ohmiya, Y. Toya and F. Tsuji, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 9584–9587 CrossRef CAS.
- E. M. Thompson, P. Adenot, F. Tsuji and J.-P. Renard, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 1317–1321 CrossRef CAS.
- G. Miesenbock and J. E. Rothman, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 3402–3407 CrossRef CAS.
- T. Suzuki, S. Usuda, H. Ichinose and S. Inouye, FEBS Lett., 2007, 581, 4551–4556 CrossRef CAS PubMed.
- T. Suzuki, C. Kondo, T. Kanamori and S. Inouye, Anal. Biochem., 2011, 415, 182–189 CrossRef CAS PubMed.
- T. Suzuki, C. Kondo, T. Kanamori and S. Inouye, PLoS One, 2011, 6, e25243 CAS.
- S. Inouye and Y. Sahara, Biochem. Biophys. Res. Commun., 2008, 365, 96–101 CrossRef CAS PubMed.
- T. Kimura, K. Hiraoka, N. Kasahara and C. R. Logg, J. Gene Med., 2010, 12, 528–537 CrossRef CAS PubMed.
- S. Inouye, Y. Sahara-Miura, J.-I. Sato, R. Iimori, S. Yoshida and T. Hosoya, Protein Expression Purif., 2013, 88, 150–156 CrossRef CAS PubMed.
- D. Morse and B. A. Tannous, Mol. Ther., 2012, 20, 692–693 CrossRef CAS PubMed.
- R. Kojima, H. Takakura, T. Ozawa, Y. Tada, T. Nagano and Y. Urano, Angew. Chem., Int. Ed., 2012, 51, 1–6 CrossRef.
- O. Shimomura, B. Musick and Y. Kishi, Biochem. J., 1988, 251, 405–410 CAS.
- O. Shimomura, B. Musick and Y. Kishi, Biochem. J., 1989, 261, 913–920 CAS.
- C. F. Qi, Y. Gomi, T. Hirano, M. Ohashi, Y. Ohmiya and F. Tsuji, J. Chem. Soc., Perkin Trans. 1, 1992, 1607–1611 RSC.
- S. Inouye and O. Shimomura, Biochem. Biophys. Res. Commun., 1997, 233, 349–353 CrossRef CAS PubMed.
- C. Wu, H. Nakamura, A. Murai and O. Shimomura, Tetrahedron Lett., 2001, 42, 2997–3000 CrossRef CAS.
- M. P. Hall, J. Unch, B. F. Binkowski, M. P. Valley, B. L. Butler, M. G. Wood, P. Otto, K. Zimmerman, G. Vidugiris, T. Machleidt, M. B. Robers, H. A. Benink, C. T. Eggers, M. R. Slater, P. L. Meisenheimer, D. H. Klaubert, F. Fan, L. P. Encell and K. V. Wood, ACS Chem. Biol., 2012, 7, 1848–1857 CrossRef CAS PubMed.
- M. Adamczyk, D. D. Johnson, P. G. Mattingly, Y. Pan and R. E. Reddy, Org. Prep. Proced. Int., 2001, 33, 477–485 CrossRef CAS.
- M. Adamczyk, S. R. Akireddy, D. D. Johnson, P. G. Mattingly, Y. Pan and R. E. Reddy, Tetrahedron, 2003, 59, 8129–8142 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- S. B. Kim, H. Suzuki, M. Sato and H. Tao, Anal. Chem., 2011, 83, 8732–8740 CrossRef CAS PubMed.
- M. H. Degeling, M. S. S. Bovenberg, G. K. Lewandrowski, M. C. de Gooijer, C. L. A. Vleggeert-Lankamp, M. Tannous, C. A. Maguire and B. A. Tannous, Anal. Chem., 2013, 85, 3006–3012 CrossRef CAS PubMed.
- S. Inouye and Y. Sahara, Biochem. Biophys. Res. Commun., 2008, 365, 96–101 CrossRef CAS PubMed.
- G. Tzertzinis, E. Schildkraut and I. Schildkraut, PLoS One, 2012, 7, e40099 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis and characterization of coelenterazine, 2-BnO-TEG-CTZ, 6-BnO-TEG-CTZ, and CoelPhos. In addition, details of generation of GLucM23 is included. See DOI: 10.1039/c3sc51985f |
| This journal is © The Royal Society of Chemistry 2013 |
