Electrophilic α-oxygenation reaction of β-ketoesters using N-hydroxycarbamates: control of the ambident reactivity of nitrosoformate intermediates†
Abstract
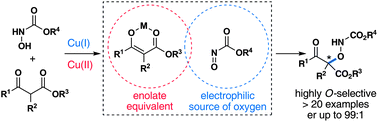
A copper-catalyzed aminooxylation of β-ketoesters using transient nitrosoformate intermediates is reported. The transformation is highly practical, efficient and highlights the ambident reactivity of nitrosocarbonyl compounds through a rare example of a nitrosocarbonyl aldol reaction. Along with a broad substrate scope, the reaction conditions that help control the regiochemistry are explored and the use of N-carbamate protected

 Please wait while we load your content...
Please wait while we load your content...