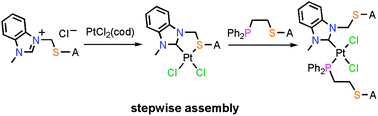
Herein we demonstrate a stepwise synthesis of heteroligated PtII Weak-Link Approach complexes with hemilabile N-heterocyclic carbene–thioether (NHC,S) and phosphino-thioether (P,S) ligands. These complexes, with both tweezer and triple-layer geometries, can be toggled between open, semiopen, and condensed states through the abstraction or introduction of Cl−. All species were fully characterized by multinuclear NMR spectroscopy and, in many cases, by single-crystal X-ray diffraction studies. Because the condensed tweezer and triple-layer species exhibit dynamic behavior at room temperature, the tweezer complex was studied by variable temperature NMR spectroscopy, revealing two species at low temperatures thought to be diastereomers resulting from thioether inversion. The relevant thermodynamic parameters for this exchange were determined. Competition experiments were performed to probe the lability of the P,S ligand, and the ligand scrambling that occurs in WLA systems with entirely P,S ligands was not observed in these NHC,S/P,S heteroligated complexes. In contrast to the halide-induced ligand rearrangement reaction, in which heteroligated complexes form when the electron donating abilities of the “weak links” are different, the stepwise assembly strategy described here does not require different electron-donating abilities of the “weak links”, as it makes use of a non-labile N-heterocyclic carbene–metal interaction instead.

 Please wait while we load your content...
Please wait while we load your content...