 Open Access Article
Open Access ArticleSelective carbohydrate–lectin interactions in covalent graphene- and SWCNT-based molecular recognition systems†
Maria-Eleni Ragoussia, Santiago Casadob, Renato Ribeiro-Vianac, Gema de la Torre*a, Javier Rojo*c and Tomás Torres*ab
aDepartamento de Química Orgánica, Universidad Autónoma de Madrid, 28049 Madrid, Spain. E-mail: tomas.torres@uam.es; Fax: +34 9 1497 3966; Tel: +34 9 1497 4151
bIMDEA-Nanociencia, c/Faraday 9, Campus de Cantoblanco, 28049 Madrid, Spain
cGlycosystems Laboratory, Instituto de Investigaciones Químicas (IIQ) CSIC – Universidad de Sevilla Americo Vespucio, 49, 41092 Sevilla, Spain. E-mail: javier.rojo@iiq.csic.es; Fax: +34 954460165; Tel: +34 954489568
First published on 2nd August 2013
Abstract
Two new protein recognition systems have been prepared by covalent functionalization of few-layer graphene and SWCNTs with α-D-mannosyl dendrons. A glycodendron, bearing three mannose moieties on the periphery for effective interaction with the lectin, and an azido group at the focal position, has been connected, by means of a “click” chemistry reaction, to both few-layer graphene and SWCNTs endowed with terminal ethynyl groups. The specific affinity of these systems for concanavalin A was thoroughly investigated by AFM, fluorescence and UV-Vis studies. A very reliable approach was used during the AFM investigation of this kind of hybrids; namely, we were able to capture the same object before and after treatment with ConA, which allowed us to obtain reliable conclusions, in terms of selectivity of the carbohydrate–lectin interactions over the nanocarbon platforms.
Introduction
Carbon allotropes – fullerenes, carbon nanotubes and, more recently, graphene – are considered as some of the most important materials in the 21st century. For some years, carbon nanotubes, and especially single-walled carbon nanotubes (SWCNTs), were in the vanguard of research owing to their outstanding electronic and mechanical properties,1,2 but, nowadays, graphene and graphene-based materials have surpassed them since they offer a number of fundamentally superior qualities that render them very promising for a wide range of applications, particularly in electronic devices.3,4 The vast potential of this carbon nanomaterial has motivated its implementation into a wide array of devices.5,6 Ideally, graphene is a single-layer material, but samples with two or more layers (i.e. few-layer graphene, FLG) are being investigated with equal interest.Graphene is nowadays attracting increasing interest for the development of highly sensitive chemical7,8 and biological sensors9,10 due to its unique combination of several important characteristics, namely, its single-atom thickness and inherently low electrical noise that enable ultrasensitive detection of many chemical and biological molecules, including proteins.11
Lectins are proteins that bind mono- and oligosaccharides with high specificity, and play an important role in biological recognition events.12 Carbohydrate–lectin interactions are a crucial step in many biological processes, such as inflammation, embryogenesis, cell development, tumor progression, etc.13 Carbohydrate–lectin binding has dissociation constants in the millimolar range, thus, multivalent systems are required for efficient recognition (simultaneous binding of multiple ligands on to multiple receptors).14–16 In an effort to understand and mimic these biological phenomena, the preparation of multivalent systems, in which various copies of the carbohydrate ligand are present, is hence essential.
In recent years, there has been a plethora of systems reported for the investigation of carbohydrate–lectin interactions.17–20 Among them, sugar-functionalized CNTs have received considerable attention, in non-covalent21–26 as well as covalent systems,21,27–30 since they are ideal platforms for the construction of multivalent architectures.27,28 In the case of graphene-based ensembles, however, examples are scarce. To the best of our knowledge, only two non-covalent systems have been reported,22,31 and regarding covalent approaches, no systems have been developed to date. Besides that, non-modified few layer graphene obtained by solvent assisted exfoliation of graphite32,33 has never been used for the construction of molecular recognition systems.
Taking advantage of our broad experience in functionalizing CNTs34,35 and graphene33 with electroactive moieties in the search for hybrid systems for electronic applications, as well as of our background in the study of carbohydrate-based molecular recognition systems,36–38 we have prepared novel hybrids for the investigation of carbohydrate–lectin interactions. We, thus, describe herein a new molecular recognition system based on non-modified graphene, as well as a related SWCNT-based ensemble, covalently linked to mannose–glycodendrons. We opted for the covalent instead of supramolecular binding of our carbohydrate ligands to the surface of the nanocarbon materials, taking into consideration the major advantages of this approach, namely, greater stability of the hybrid material, control over the degree of functionalization, and reproducibility. The specific affinity of these systems for concanavalin A (ConA) was thoroughly investigated as a proof of concept to demonstrate the viability of these platforms for bio-sensing applications.
Results and discussion
We have used a glycodendron bearing three mannose moieties on the periphery, for effective interaction with the lectin, and an azido group at the focal position for carrying out a “click” chemistry reaction to connect it to carbon nanostructures endowed with terminal ethynyl groups. The synthesis of glycodendron 5 was achieved in four steps, starting from pentaerythritol, by a procedure described in the literature (Scheme S1 in the ESI†).36,39 Briefly, the trialkynylated compound 1 was prepared from commercially available pentaerythritol by reaction with propargyl bromide and sodium hydride. Then, a short, conveniently functionalized spacer was conjugated to the fourth hydroxyl group giving rise to compound 2. The mannosyl units were incorporated to the core by means of Cu(I) catalyzed azide–alkyne cycloaddition (CuAAC), using mannosyl derivative 3 previously synthesized in our group40 to yield trivalent dendron 4. Finally, substitution of the chloro by an azido group provided compound 5, ready to be clicked on the conveniently functionalized surfaces.At the same time, graphene, and also SWCNTs were initially functionalized with a phenylethynyl group, to install the required alkyne for the CuAAC reaction. In the case of SWCNTs, microwave irradiation of a well-dispersed batch of carbon nanotubes in o-DCB, in the presence of isoamyl nitrite and 4-[(trimethylsilyl)ethynyl]aniline,41 afforded derivative 6 (Scheme 1). Thermogravimetric analysis (TGA) showed that microwave irradiation led to a significantly higher degree of functionalization when compared to thermal conditions previously used by us (Fig. 1a).34 More specifically, the number of phenylacetylene groups in 6 were estimated to be 1 per 70 carbon atoms at 600 °C (17% weight loss), more than double of what we had previously achieved (8%). In addition, a much lower reaction time, 1.5 instead of 48 h, was required. Derivative 6 was subsequently treated with TBAF to remove the protecting TMS group and was then immediately subjected to a Cu(I)-catalyzed 1,3-dipolar cycloaddition with trivalent dendron 5, to afford final hybrid 7 (Scheme 1). Taking into consideration the total decomposition of 5 at 600 °C (86%), an average functionalization of 1 glycodendron per ca. 170 carbon atoms (35% weight loss) was calculated (Fig. 1a). It is noteworthy that the additional weight loss of hybrid 7 with respect to that of phenylethynyl–SWCNT precursor 6 was detected at a temperature above 300 °C, which matches the weight loss temperature of the mannosyl dendron 5 (Fig. 1a), that being strong evidence for the linkage of the dendron to the carbon nanostructure.
![Reagents and conditions: (a) 4-[(trimethylsilyl)ethynyl]aniline, isoamyl nitrite, o-DCB, MW, 80 °C, 1.5 h; (b) TBAF, 0 °C to rt, 2 h; (c) dendron 5, CuSO4, sodium ascorbate, 70 °C, 48 h; (d) 4-[(trimethylsilyl)ethynyl]aniline, isoamyl nitrite, NMP, MW, 80 °C, 1.5 h.](/image/article/2013/SC/c3sc51352a/c3sc51352a-s1.gif) | ||
| Scheme 1 Reagents and conditions: (a) 4-[(trimethylsilyl)ethynyl]aniline, isoamyl nitrite, o-DCB, MW, 80 °C, 1.5 h; (b) TBAF, 0 °C to rt, 2 h; (c) dendron 5, CuSO4, sodium ascorbate, 70 °C, 48 h; (d) 4-[(trimethylsilyl)ethynyl]aniline, isoamyl nitrite, NMP, MW, 80 °C, 1.5 h. | ||
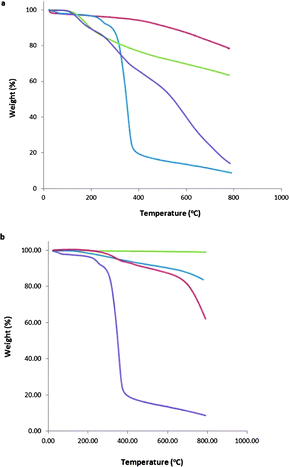 | ||
| Fig. 1 TGA for (a) SWCNT-based hybrids (red line: pristine SWCNTs, green line: conjugate 6, purple line: conjugate 7, blue line: glycodendron 5) and (b) graphene-based hybrids (green line: exfoliated graphene, blue line: conjugate 8, red line: conjugate 9, purple line: glycodendron 5). | ||
Regarding glycographene system 9, its preparation differed slightly to that described above, since the first step involved exfoliation of graphite to obtain FLG, using the well-known method of liquid exfoliation in NMP.32,33 The suspension of FLG was immediately subjected to microwave irradiation to install the necessary alkyne, and was followed by deprotection and “click” reaction to link the carbohydrate dendron (Scheme 1). TGA experiments revealed that a significantly lower degree of functionalization was achieved, when compared to SWCNT-based hybrid 7, which was expected considering the minor reactivity of this carbon nanomaterial. In particular, we calculated 9% weight loss at the initial functionalization step (hybrid 8), which corresponds to 1 phenylacetylene group per ca. 150 carbon atoms. After implementation of the sugars, an extra 6% weight loss (when considering the total decomposition of 5 at 600 °C) was observed, corresponding to 1 glycodendron per ca. 1500 carbon atoms (Fig. 1b).
The solubility/dispersability of SWCNT- and graphene-glycodendron hybrids 7 and 9 was tested in various solvents. Even though water solubility is desirable for biological applications, the attachment of mannose-based dendrons does not lead to water-soluble materials. Nevertheless, nanoconjugate 9 forms very stable suspensions in DMSO (Fig. S1 in ESI†) which remain stable after five days, whereas those of both protected graphene–phenylethynyl system 8 and exfoliated graphene show a black precipitate after that period of time. The stability and homogeneity of the dispersions of 9 allowed us to carry out 1H NMR experiments in DMSO-d6. From the spectrum (Fig. S2 in ESI†) we obtained irrefutable proof for the covalent linkage of the glycodendron to the modified graphene surface, since a new singlet appeared at 8.31 ppm, which corresponds to the proton of the triazole ring formed in the latter “click” chemistry reaction between phenylethynyl-functionalized graphene precursor 8 and glycodendron 5. This signal integrates perfectly with the signal at 7.95 ppm (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio), which corresponds to the pre-existing triazole rings of the glycodendron (Fig. S2 in ESI†). Unfortunately, the quality of the dispersions of the related SWCNT-based hybrid 7 in different solvents was too low to perform NMR characterization of this material.
3 ratio), which corresponds to the pre-existing triazole rings of the glycodendron (Fig. S2 in ESI†). Unfortunately, the quality of the dispersions of the related SWCNT-based hybrid 7 in different solvents was too low to perform NMR characterization of this material.
With the two new systems (i.e.7 and 9) in hand, we proceeded to the study of their interaction with a lectin that selectively binds mannose. Among lectins, the most widely used in affinity studies is the commercially available concanavalin A (ConA) from Canavalia ensiformis (purified from jack-bean). ConA is a tetrameric lectin (at pH 7.0 or above) that selectively binds mannose and glucose. For maintaining the loop-type active conformation and establishing effective sugar-binding activity, it requires the presence of divalent cations, most specifically, calcium and/or manganese.42
Atomic force microscopy (AFM) was the tool we used to obtain initial proof for the successful functionalization of the carbon nanostructures with glycoconjugate 5, and then for the selective interaction of the hybrids with ConA. All measurements were performed on poly-lysine treated mica surfaces, this treatment allowing the carbon nanostructures to stick robustly to the surface.
Concerning SWCNTs, as shown in Fig. 2a, a height of approximately 1 nm was measured for derivative 6, bearing the alkyne moieties. After installing the mannose glycoconjugates, the height was raised to ca. 5 nm (Fig. 2b). In addition, zooming into one nanotube (Fig. S3a in ESI†), we could observe higher and lower parts, which can be attributed to the dendron being randomly implemented around the nanotube, where the alkynes were originally installed, as already demonstrated by 1H NMR (Fig. S2 in ESI†). The estimated length of the glycodendron excluding the ethyleneglycol linker is 1.5 nm.36 Considering that the dendron units can be randomly located along the whole nanotube, namely in the lower part stuck to the surface and also in the upper part, the observed increase fits quite well with that expected for these systems. Similarly, imaging a sample of FLG bearing the alkyne groups, the height was of the order of 4–8 nm, whereas in hybrid 9 there was an average increase of around 6–8 nm (Fig. S3b and c in ESI†). In this case, the calculated increase in height after installation of the dendron does not necessarily fit with the observed value, considering that each flake imaged may have a different number of layers. It should also be pointed out that the relatively large height values found for the FLG are consistent, not only with the presence of several graphene layers, but also with the presence of glycodendron protuberances which do not allow for a fully planar deposition of the graphene flakes on the substrate even if the functionalization is only random along the surface.
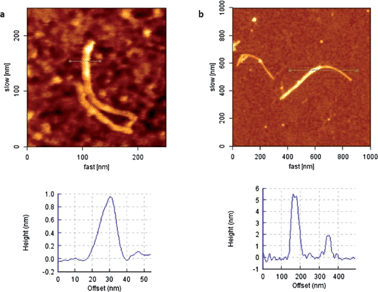 | ||
| Fig. 2 (a) SWCNT before installation of carbohydrate dendron (6) and height profile and (b) SWCNT after installation of carbohydrate dendron (7) and height profile. Please note that the highest profile (ca. 5 nm) would correspond to the location of dendritic units in the “lower” and in the “upper” positions of the tube; and the lowest profile (2 nm) would correspond to a region where no dendrons are attached to the top. | ||
At this point, for the investigation of the affinity of SWCNT– and graphene–carbohydrate hybrids with ConA, we adopted a reliable strategy for measuring this selective interaction. In contrast to other works reported so far, we imaged the exact same CNT or graphene item before and after treatment with ConA. Focusing on the same nanotube or flake and measuring the height at the same position before and after the interaction with the lectin, the evidence of sugar–protein recognition is much more accurate.
Hence, we set off our interaction studies by drop-casting an ethanol dispersion of graphene-hybrid 9 to a poly-lysine-treated mica surface, and obtained an image of one FLG flake (Fig. 3a and Fig. S4 in ESI†) with a height of around 10 nm. Maintaining the area of measurement, we incubated the surface with a 0.2 mg mL−1 solution of ConA in phosphate buffered saline (PBS), in the presence of either Mn2+ or Ca2+ ions, for 45 min. It should be pointed out that Mn2+ ions augment the lectin–carbohydrate interaction compared to Ca2+, as stated in a recent report17 and indeed, incubation in the presence of Ca2+ did not afford conclusive results. Hence, we continued our studies using Mn2+. We took images in the presence of the lectin solution (Fig. 3b and S4 in ESI†) because, in this way, we could avoid ConA aggregates on the mica, as well as on the FLG flakes, which could cause difficulties and inaccuracies in the study of the selective interaction process. Under these conditions, an increase in height of ca. 10 nm was observed. The size of ConA at pH 7 or higher, as determined by X-ray diffraction studies, is 60 × 70 × 70 Å. Although the height increase of the FLG flake is somewhat larger than the size of ConA, the measured value fully fits with that previously reported for graphene materials non-covalently linked to related glycoconjugates, which interact with ConA.22 To demonstrate that a selective binding with the mannose moieties is taking place, we performed a control experiment with non-specific protein, BSA (albumin from bovine serum). By the same procedure, focusing on one FLG flake, we carried out the incubation of the mica surface with a solution of BSA at the same concentration (i.e. 0.2 mg mL−1) in PBS in the presence of Mn2+ and then measured the same flake again. As assumed, no significant change in height was observed in this case (Fig. 3c and d). This observation proved the selectivity of our system and also allowed us to discard that the height differences observed in the experiment with ConA were a consequence of roughness differences on the background due to direct ConA unspecific adhesion to the treated mica.
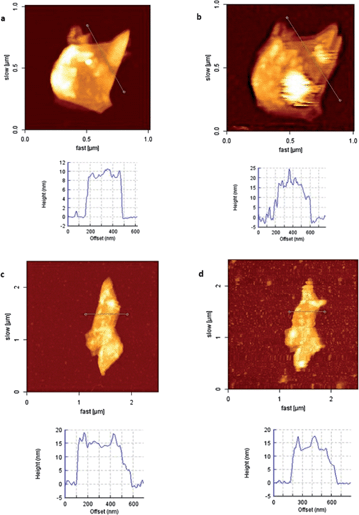 | ||
| Fig. 3 (a) Graphene–carbohydrate 9 before incubation with ConA and height profile, (b) graphene–carbohydrate 9 after incubation with ConA for 45 min, and height profile, (c) graphene–carbohydrate 9 before incubation with BSA and height profile, and (d) graphene–carbohydrate 9 after incubation with BSA for 45 min and height profile. | ||
For the nanotube-based systems, the same sequence of experiments was applied. After drop-casting an ethanol dispersion of SWCNT-hybrid 7 to a treated mica surface, we followed the height changes on some selected nanotubes (Fig. 4a and S5 in ESI†) with a height of 4 nm at their highest part. In this case, we could not take well-resolved images of the nanotubes in the presence of the lectin solution, probably because their interaction with the treated surface is lower in these conditions. This could be a consequence of the lower number of contact points when compared to FLG flakes, which are more planar and less functionalized (see the functionalization degree obtained by TGA measurements).
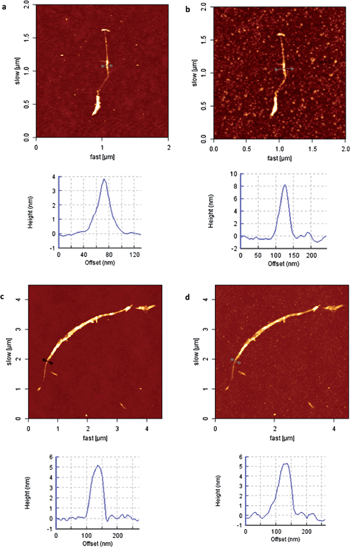 | ||
| Fig. 4 (a) SWCNT–carbohydrate 7 before incubation with ConA and height profile, (b) SWCNT–carbohydrate 7 after incubation with ConA for 45 min and height profile, (c) SWCNT–carbohydrate 7 before incubation with BSA and height profile, and (d) SWCNT–carbohydrate 7 after incubation with BSA for 45 min and height profile. | ||
Thus, imaging of the nanotubes was carried out in air. In particular, after incubation and subsequent washing with water to remove excess lectin, we captured images of the exact same nanotube (Fig. 4b and S5b in ESI†). An increase in height of 4 nm was observed in the swollen parts of the nanotube (Fig. 4b and S5b in ESI†), whilst the thinner parts remained unaltered (Fig. S5c and d in ESI†). The increase in height fully fits, in this case, with the ConA dimensions. Finally, a reference experiment with BSA was also conducted for SWCNT-hybrid 7, and once again, no change in height was observed, as demonstrated in Fig. 4c and d.
To further corroborate the affinity of our systems for ConA, we decided to perform fluorescence and UV-Vis experiments using fluorescently labeled lectin ConA-Alexa488. In particular, fluorescence evaluation was not possible for FLG nanohybrid 9 due to the well-known fluorescence-quenching effect of graphene,43 and only UV-vis spectra over dispersions of this material could be performed (see below). To conjugate ConA with hybrid systems 7 and 9, fine dispersions of the nanoconjugates in EtOH/PBS were treated with a solution of ConA-Alexa488 in PBS and incubated for 1 h. Centrifugation and washing with PBS, to remove excess of the lectin, afforded the modified materials, in which the fluorescent lectin would be fixed at the mannose residues. Fluorescence and AFM coupled images of dispersions of the fluorescent-ConA-treated SWCNT-hybrid 7 on mica are depicted in Fig. S6 in ESI.† The fluorescence image displays some fluorescent spots in the same positions as the bundles of nanotubes in the AFM image. This observation confirms the preference of the lectin for the modified nanotube, since no trace of fluorescence could be observed in the rest of the surface. In addition, UV-Vis experiments over dispersions of the modified hybrid 7 in PBS showed the signature of the fluorescent ConA at 490 nm (Fig. 5). In contrast, when incubating pristine SWCNTs with ConA, no apparent trace of the fluorescent lectin was observed. Similarly, the interaction of graphene-conjugate 9 with fluorescent ConA is clearly visible in the UV-vis spectrum of the fluorescent-ConA-treated hybrid 9, whereas no interaction was observed when non-functionalized, exfoliated graphene was incubated with the lectin (Fig. S7 in ESI†).
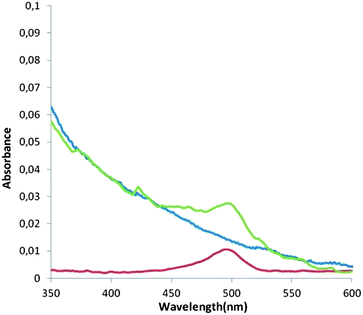 | ||
| Fig. 5 UV-Vis spectrum of ConA-Alexa488 in PBS (red line), SWCNT-glycoconjugate 7 incubated in ConA-Alexa488 in PBS (green line) and bare SWCNT incubated in ConA-Alexa488 in PBS (blue line). | ||
Conclusions
In conclusion, we have prepared two new FLG- and SWCNT-based protein recognition systems, by covalent functionalization with trivalent α-D-mannosyl dendrons. These systems demonstrated selective interaction with ConA, as proved by AFM, fluorescence and UV-Vis studies. The key features of this work rely on three crucial points. First, the covalent linkage of the glycodendrons to the nanocarbon materials afforded novel and robust systems for this type of studies. Second, this is the first report on non-modified graphene being used for functionalization with carbohydrates and biological studies. And, lastly, a novel approach was used during the AFM investigation of this kind of hybrids, namely, we succeeded in capturing the same object before and after treatment with ConA, which allowed us to obtain highly reliable results, in terms of selectivity of carbohydrate–lectin interactions over the nanocarbon platforms. Concerning future applications of the herein described architectures, they show high potential for their incorporation in biosensors, as well as drug-delivery systems.Experimental
General
1H NMR spectra were recorded at 300 MHz at 25 °C with a BRUKER AC-300 instrument. UV-Vis spectra were recorded with a JASCO V-660 spectrophotometer. Thermogravimetric analyses were performed with a thermobalance TGA Q500 TA Instrument equipped with an EGA oven at 10 °C min−1 under N2 (90 mL min−1 flux). Atomic force microscopy (AFM) characterization was performed using a JPK NanoWizard II, coupled to a Nikon Eclipse Ti inverted fluorescence optical microscope. An Olympus commercial silicon nitride cantilever tip (OMCL-RC800PSA) with a force constant of 0.76 N m−1 and a resonant frequency of 71 kHz was employed in dynamic mode.Materials
Graphite flakes (+100 mesh, ≥75% min) were purchased from Aldrich, as well as all other chemicals, except HiPco SWCNTs, purchased from Carbon Nanotechnologies Inc., and fluorescent ConA from Invitrogen.Preparation of AFM samples
Freshly peeled mica was submerged in a 0.001% poly-lysine solution for 1 min, then rinsed with water and dried with Ar. A dispersion of the graphene sample in EtOH was then drop-casted onto the coated mica. After evaporation of the solvent, the samples were investigated. For the incubation with specific and non-specific lectins, a solution of the corresponding lectin (c = 0.2 mg mL−1) in PBS in the presence of MnBr2 (c = 1 mg mL−1) was drop-casted on the mica surface and incubated for 45 min. In the case of the SWCNTs, after incubation the mica surface was washed with water and dried in air.Synthesis
Acknowledgements
Financial support is acknowledged from the Spanish MICINN (CTQ2011-24187/BQU, CTQ2011-23410/BQU and CONSOLIDER INGENIO 2010, CSD2007-00010 on Molecular Nanoscience) and the Comunidad de Madrid (MADRISOLAR-2, S2009/PPQ/1533). Thanks to the subscription of Red de Bibliotecas del CSIC to the RSC Gold for Gold Application for getting open access for this article.Notes and references
- S. Reich, C. Thomsen and J. Maultzsh, Carbon Nanotubes: Basic Concepts and Physical Properties, VCH, Weinheim, 2004 Search PubMed.
- V. Sgobba and D. M. Guldi, Chem. Soc. Rev., 2009, 38, 165 RSC.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666 RSC.
- N. O. Weiss, H. Zhou, L. Liao, Y. Liu, S. Jiang, Y. Huang and X. Duan, Adv. Mater., 2012, 24, 5782 CrossRef CAS.
- X. Huang, Z. Zeng, Z. Fan, J. Liu and H. Zhang, Adv. Mater., 2012, 24, 5979 CrossRef CAS.
- K. R. Ratinac, W. Yang, S. P. Ringer and F. Braet, Environ. Sci. Technol., 2010, 44, 1167 CrossRef CAS.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652 CrossRef CAS.
- H. Jiang, Small, 2011, 7, 2413 CAS.
- Y. Liu, X. Dong and P. Chen, Chem. Soc. Rev., 2012, 41, 2283 RSC.
- S. Mao, G. Lu, K. Yu, Z. Bo and J. Chen, Adv. Mater., 2010, 22, 3521 CrossRef CAS.
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637 CrossRef CAS.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357 CrossRef CAS.
- M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754 CrossRef.
- Y. C. Lee and R. T. Lee, Acc. Chem. Res., 1995, 28, 321 CrossRef CAS.
- P. I. Kitov and D. R. Bundle, J. Am. Chem. Soc., 2003, 125, 16271 CrossRef CAS.
- B. K. Gorityala, Z. Lu, M. L. Leow, J. Ma and X. W. Liu, J. Am. Chem. Soc., 2012, 134, 15229 CrossRef CAS.
- J.-F. Nierengarten, J. Iehl, V. Oerthel, M. Holler, B. M. Illescas, A. Muñoz, N. Martín, J. Rojo, M. Sánchez-Navarro, S. Cecioni, S. Vidal, K. Buffet, M. Durka and S. P. Vincent, Chem. Commun., 2010, 46, 3860 RSC.
- N. Jayaraman, Chem. Soc. Rev., 2009, 38, 3463 RSC.
- B. T. Houseman and M. Mrksich, Top. Curr. Chem., 2002, 218, 1 CrossRef CAS.
- Y. Chen, A. Star and S. Vidal, Chem. Soc. Rev., 2013, 42, 4532 RSC.
- Y. Chen, H. Vedala, G. P. Kotchey, A. Audfray, S. Cecioni, A. Imberty, S. Vidal and A. Star, ACS Nano, 2012, 6, 760 CrossRef CAS.
- H. Vedala, Y. Chen, S. Cecioni, A. Imberty, S. Vidal and A. Star, Nano Lett., 2011, 11, 170 CrossRef CAS.
- T. Hasegawa, T. Fujisawa, M. Numata, M. Umeda, T. Matsumoto, T. Kimura, S. Okumura, K. Sakurai and S. Shinkai, Chem. Commun., 2004, 2150 RSC.
- N. Khiar, M. P. Leal, R. Baati, C. Ruhlmann, C. Mioskowski, P. Schultz and I. Fernandez, Chem. Commun., 2009, 4121 RSC.
- K. S. Vasu, K. Naresh, R. S. Bagul, N. Jayaraman and A. K. Sood, Appl. Phys. Lett., 2012, 101, 053701 CrossRef.
- P. G. Luo, H. Wang, L. Gu, F. Lu, Y. Lin, K. A. Christensen, S. T. Yang and Y. P. Sun, ACS Nano, 2009, 3, 3909 CrossRef CAS.
- L. Gu, T. Elkin, X. Jiang, H. Li, Y. Lin, L. Qu, T.-R. J. Tzeng, R. Joseph and Y.-P. Sun, Chem. Commun., 2005, 874 RSC.
- H. Wang, L. Gu, Y. Lin, F. Lu, M.-J. Meziani, P.-G. Luo, W. Wang, L. Cao and Y.-P. Sun, J. Am. Chem. Soc., 2006, 128, 13364 CrossRef CAS.
- C. Gao, S. Muthukrishnan, W. Li, J. Yuan, Y. Xu and A. H. E. Muller, Macromolecules, 2007, 40, 1803 CrossRef CAS.
- Q. Chen, W. Wei and J.-M. Lin, Biosens. Bioelectron., 2011, 26, 4497 CrossRef CAS.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563 CrossRef CAS.
- M.-E. Ragoussi, J. Malig, G. Katsukis, B. Butz, E. Spiecker, G. de la Torre, T. Torres and D. M. Guldi, Angew. Chem., Int. Ed., 2012, 51, 6421 CrossRef CAS.
- S. Campidelli, B. Ballesteros, A. Filoramo, D. Diaz Diaz, G. de la Torre, T. Torres, G. M. Aminur Rahman, C. Ehli, D. Kiessling, F. Werner, V. Sgobba, D. M. Guldi, C. Cioffi, M. Prato and J.-P. Bourgoin, J. Am. Chem. Soc., 2008, 130, 11503 CrossRef CAS.
- B. Ballesteros, G. de la Torre, C. Ehli, G. M. A. Rahman, F. Agulló-Rueda, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2007, 129, 5061 CrossRef CAS.
- R. Ribeiro-Viana, M. Sánchez-Navarro, J. Luczkowiak, J. R. Koeppe, R. Delgado, J. Rojo and B. G. Davis, Nat. Commun., 2012, 3, 1303 CrossRef.
- J. Luczkowiak, A. Muñoz, M. Sánchez-Navarro, R. Ribeiro-Viana, A. Ginieis, B. M. Illescas, N. Martín, R. Delgado and J. Rojo, Biomacromolecules, 2013, 14, 431 CrossRef CAS.
- M. Sánchez-Navarro, A. Muñoz, B. M. Illescas, J. Rojo and N. Martín, Chem.–Eur. J., 2011, 17, 766 CrossRef.
- M. Ortega-Muñoz, J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Adv. Synth. Catal., 2006, 348, 2410 CrossRef.
- E. Arce, P. M. Nieto, V. Díaz, R. G. Castro, A. Bernad and J. Rojo, Bioconjugate Chem., 2003, 14, 817 CrossRef CAS.
- J.-J. Hwang and J. M. Tour, Tetrahedron, 2002, 58, 10387 CrossRef CAS.
- K. D. Hardman and C. F. Ainsworth, Biochemistry, 1972, 11, 4910 CrossRef CAS.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure followed for glycodendron 5, photos of dispersions of the graphene-based materials 8 and 9, 1H NMR spectrum of graphene–dendron hybrid 9 and AFM images of functionalized SWCNTs and FLG before and after their interaction with ConA. See DOI: 10.1039/c3sc51352a |
| This journal is © The Royal Society of Chemistry 2013 |
