Representations of chemical bonding models in school textbooks – help or hindrance for understanding?
Anna Bergqvist*a,
Michal Drechslera,
Onno De Jongb and
Shu-Nu Chang Rundgrena
aKarlstad University, Chemistry, Universitetsgatan, 2, Karlstad, Sweden. E-mail: anna.bergqvist@kau.se
bUtrecht University, Oosterlaan 51, Driebergen, Netherlands
First published on 16th July 2013
Abstract
Models play an important and central role in science as well as in science education. Chemical bonding is one of the most important topics in upper secondary school chemistry, and this topic is dominated by the use of models. In the past decade, research has shown that chemical bonding is a topic that students find difficult, and therefore, a wide range of alternative conceptions are developed by students. This study focuses on analyzing the models of chemical bonding in chemistry textbooks at upper secondary level and aims to investigate the content of chemical bonding presented in chemistry textbooks related to students' alternative conceptions and difficulties in understanding. Chapters concerning chemical bonding in five chemistry textbooks at upper secondary level in Sweden were analyzed. The results showed that the models of chemical bonding represented in the school textbooks might cause students to have alternative conceptions and difficulties in understanding chemical bonding, which matched the findings found by other recent studies. Thereby, the results indicate a need for filling in the gap between research and textbook writers. Implications for textbook authors and teachers are addressed.
Introduction
In science, natural phenomena in the world as-experienced are described and predicted, and explanations are found (Gericke and Hagberg, 2007). These outcomes of science are concepts, models and theories. Concepts can be described as entities of which the world is believed to consist of; models as proposals for how these concepts physically and temporally correlate to each other in the material world; and theories as general sets of reasons why these concepts and models can be thought to occur (Gilbert et al., 2000). In the study of science, understanding the concepts that shape science is important, and models play an important role when scientific knowledge is developed and when science is communicated. Chemical bonding is one of the most important topics taught in chemistry at upper secondary school level, and this topic is dominated by the use of models (Taber and Coll, 2002). However, the use of models in science education is not unproblematic. Students' difficulties in understanding models in general have been reported in research, and research has also shown that the use of models in science education can cause students' difficulties in understanding. For instance, students regard models as exact replicas of the real thing (Grosslight et al., 1991; Ingham and Gilbert, 1991). Furthermore, students might be confused when new models are introduced, and then combine attributes from different models (Justi and Gilbert, 2002a). Regarding chemical bonding, during the past decade, research has shown that chemical bonding is a topic that students find difficult, and a wide range of alternative conceptions are developed by students within this topic (reviewed by Taber and Coll, 2002; Özmen, 2004).In science education, the models are presented for the students mainly by the textbooks and the teachers. Presentations in textbooks as well as teachers' teaching influence the students' knowledge and understanding (Yager, 1983; Tulip and Cook, 1993; Sikorova, 2012). The frequency of influence of textbooks on the content of lessons is significantly high in science education (Roth et al., 2006), and in chemical education, the textbook is the most widely and frequently used teaching aid (Justi and Gilbert, 2002b). The models presented in the textbooks can cause students' difficulties in understanding, as shown by, for instance, Gericke and Hagberg (2010b) in the context of genetics. They found that the models presented in textbooks were correlated to the alternative conceptions held by students. Furthermore, they indicated that if these textbooks are used as a foundation for teaching, this correlation may persist. Consequently, it is important for the textbook writers to be aware of how the models presented in textbooks might influence the students' understanding, and to connect research to textbook writers and teachers. Accordingly, analysis of textbooks with focus on the important topic of chemical bonding may provide information that can improve chemistry learning in science education.
Based upon the above-mentioned importance of models of chemical bonding and the role of textbooks, this study focuses on analysing the presentation of chemical bonding models in chemistry textbooks at upper secondary level. The aim of this study is to investigate the content of school chemistry textbooks presenting chemical bonding and related to students' difficulties in understanding (i.e. students' alternative conceptions and difficulties in understanding). In the following section, the related research literature regarding models in general, chemical bonding models, students' difficulties in understanding and the role of textbooks are described.
Models in science education
Models play an important and central role in science, and using models is important when developing scientific knowledge. Furthermore, models play an important role when science is communicated. The central role of models in science consequently gives them equal importance in science education, and models in science attain a wide variation of epistemological states: for example scientific, historical, curricular and teaching models (Gilbert, 2007). In this study, we examined the representations of teaching models of chemical bonding in school chemistry textbooks. A teaching model is a model developed to support the learning of curricular models, often used in the form of analogies or metaphors (Gilbert, 2007). Curricular models, however, are often simplified versions of modified scientific models (models for which scientists or researchers at the front line of science have reached an agreement about) and historical models (models which are replaced by a revised scientific model) (Gilbert, 2007). Yet, a teaching model should be at an optimum level of simplification, that is, kept as simple as possible while still being scientifically correct (Taber and Coll, 2002). In such a case, a teaching model provides a ground for students to develop later on in their learning process (Taber and Coll, 2002).However, there are several research findings regarding difficulties concerning the use of models in science education and chemistry education by teachers and in textbooks. For instance, often the models are described as if the models themselves were the phenomena, and the models' nature and purpose are not discussed at all (Grosslight et al., 1991). Furthermore, teachers might forget or do not even know that they are communicating a model; instead, a model is presented as if it was a proven fact rather than entities created to highlight some aspects of theories (Treagust et al., 2002). To date, students' difficulties in understanding regarding models have been discussed. For instance, students regard models as exact replicas of the real thing (Grosslight et al., 1991; Ingham and Gilbert, 1991), and students might be confused when new models are introduced, or while combining different attributes from different models (Justi and Gilbert, 2002a). In science education, it is considered useful if students have knowledge of different states of models and recognize their functions and limitations as well as the fact that a concept can be explained by several models. In this way students gain a better understanding of scientific knowledge and the nature of science (Boulter and Gilbert, 2000; Gericke and Hagberg, 2007; Drechsler and Van Driel, 2008). However, teachers and textbooks are not always explicit when using models (Gericke et al., 2012). Attributes from separate historical models with different theoretical backgrounds are transferred and merged into so called hybrid models (Gilbert, 2007). These hybrid models might be difficult to use for teaching and learning, and may confuse students (Justi and Gilbert, 2000). Nevertheless, they are often used as teaching models by teachers and presented in textbooks in science education (e.g. Drechsler and Schmidt, 2005; Gericke and Hagberg, 2010a; Gericke et al., 2012b).
Furthermore, Gilbert (2007) indicates that a complication in science education is that any state of a model can be expressed by the use of one or more of the five modes of representation, that is, the concrete mode (e.g. ball-and-stick model), the verbal mode (spoken or written), the symbolic mode, the visual mode, and the gesture mode (e.g. movements by the body). To make it even more complicated, there are many sub-modes within each above-mentioned mode that can be difficult to interpret by the students (Gilbert, 2007). In chemistry, a chemical equation can be represented in several ways, for example, magnesium + hydrochloric acid, Mg + HCl, Mg(s) + HCl(aq), or Mg(s) + H+(aq) + Cl−(aq). To interpret and move between modes is a challenge for the students, especially when they are intermingled, for instance, while using representation such as Mg + H+(aq). In this study, the models of chemical bonding were examined by analysing the verbal, symbolic and visual modes of representation of chemical bonding models.
Models of chemical bonding
Without any doubt, chemical bonding is dominated by the use of models, and these models are important to explain chemical bonding. Students need to understand models for chemical bonding to understand chemistry, because we cannot see how atoms or other particles are held together (Coll and Treagust, 2003). Models of chemical bonding, as any models, can be expressed, that is represented, in different modes of representations according to Gilbert (2007).Due to the nature of substances and the fact that physical and chemical changes of substances are derived from the interactions between atoms or charged particles as ions (Coll and Treagust, 2003), chemical bonding is one of the most important topics taught in chemistry at upper secondary school level as well as the foundation for other topics in chemistry (Nahum et al., 2008).
In this study, the models of interest are the models for ionic, covalent, polar covalent and metallic bonding, because they are the main types of chemical bonding and have been in focus for the majority of the studies regarding students' understanding of chemical bonding. The models focused in this study are those not based on quantum mechanics. Bonding between molecules, which are not always considered as chemical bonding in science literature and research, but rather as inter-molecular forces, are not the focus of this study.
Students' difficulties in understanding regarding chemical bonding models
In the following sections, examples of students' alternative conceptions and difficulties in understanding for the different types of bonding, respectively, are presented, followed by some possible sources of these difficulties regarding chemical bonding according to the research literature. In this study these research results, in addition to the results about difficulties in understanding regarding models in general, served as the base to analyze the representations of models of chemical bonding presented in school textbooks.Examples of students' difficulties in understanding
Additional alternative conceptions reported are that the ionic lattice or ionic compounds are composed of discrete molecules or ion pairs seen as molecules of the ionic compound, which seems to be quite widespread and is referred to in several studies from several countries (Taber, 1997; Barker and Millar, 2000; Taber and Coll, 2002; Othman et al., 2008; Taber et al., 2012). In addition, the conceptions that atoms are present in ionic compounds and that these atoms become ions when the compound melts are shown by Othman and co-authors (2008). Furthermore, the authors identified the conception that free electrons are produced when an ionic compound is dissolved in water, hence making aqueous ionic compounds capable of conducting electricity but not solid ionic compounds.
Regarding the concept of polar covalent bonding, Harrison and Treagust (1996) indicated that the bond polarity, shape of molecules and polarity of molecules are unclear to the students. This was also reported by Peterson et al., (1989), for instance, the students think that the polarity of a bond is dependent on the number of valence electrons in each atom involved in the bond, and that non-polar molecules form when the atoms in the molecule have similar electronegativities. The reason for this, according to Taber and Coll (2002), could be confusion over the understanding of electronegativity and presenting ionic and covalent bonding as a dichotomy. The authors also suggested the latter as the reason for the fact that students tend to see bond polarity as a characteristic of the covalent bond instead of something in between ionic and covalent bonds.
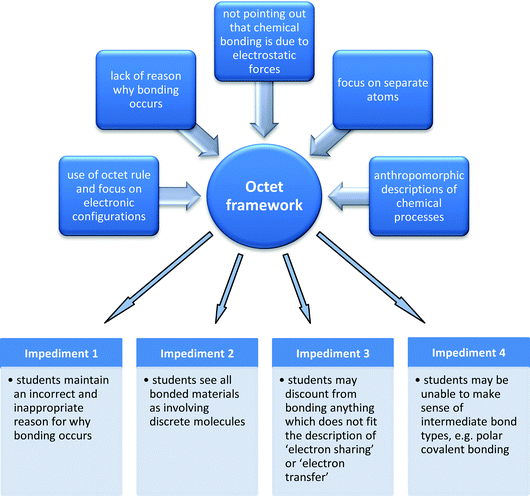 | ||
| Fig. 1 Factors that can be seen as sources for students to develop the octet framework, which provides an overarching perspective on the students' alternative conceptions that act as learning impediments. | ||
The factor ‘use of anthropomorphic description’ is common not only in the context of chemical bonding, but also in science education in general. Anthropomorphism is an extension of animism, and the term animism was defined by Piaget (1929/1973, p. 194) as ‘the tendency to regard objects as living and endowed with will.’ Namely, anthropomorphism is when human characteristics are assigned to non-living things. Although anthropomorphic explanations could be useful in the initial stages of learning about chemical bonding (and science concepts in general) they could become an impediment for students' further learning (Taber and Coll, 2002). Even if the anthropomorphic explanation is used in order to familiarize the students with abstract concepts, still, if they are used habitually, they could shift from standing-in to take the place of the explanation (Taber and Watts, 1996). In that case, students may not see a reason to develop more sophisticated explanations (Taber and Watts, 1996; Taber and Coll, 2002). Therefore, Taber and his colleagues argued that the textbooks and teachers should not use these anthropomorphic explanations uncritically.
The role of textbooks
Textbooks are as important as teachers in the process of students’ learning, and textbooks have a broad variety of functions and are used intensively in schools (Mikk, 2000). Also, they have a unique role as obligatory reading material in some countries (Ekvall, 2001). Moreover, textbooks are important not only as reading material and as a knowledge mediator, they also provide a structure for classroom activities in general (Edling, 2006). In science education, many science teachers use their textbooks in their lesson planning and classroom teaching (Tulip and Cook, 1993), and textbooks can influence the order, the meaning, the examples, and the applications of science topics (Yager, 1983). A purpose repeatedly identified in research regarding the role of textbooks is that textbooks are used as a source of the content of a lesson and as a programme for teaching (Sikorova, 2012), and the textbooks are the most thorough representation of curricula (Mikk, 2000). The frequency of influence of textbooks on the content of lesson is significantly high. For instance, in science, as the results from the TIMSS 1999 video study show, textbooks were reported by teachers as playing a major role in the teachers' decision to teach the content from 32 percent of the videotaped lessons (Australia) to as high as 67 percent (Czech Republic) and 74 percent (Netherlands) (Roth et al., 2006). Moreover, content organization during the lessons followed the outline of content in textbook to a high extent (Roth et al., 2006). Furthermore, regarding chemical education, Justi and Gilbert (2002b) mentioned the textbook as the most widely and frequently used teaching aid. In the perspective of the students, the textbooks' role in representing a source of information was pointed out by Sikorova (2012). This function, to represent information, is one of the most important functions of a textbook, and one of the main characteristics then is scientific correctness (Mikk, 2000).The importance of analyzing textbooks is crucial (Justi and Gilbert, 2002b), and it is important to evaluate textbooks in order to find out their shortcomings; otherwise, the textbooks will change slowly, and scientific research can improve the development of new and better textbooks (Mikk, 2000).
Regarding the role of textbooks in teaching and learning of models, there are several research findings indicating problems. For instance, the use of hybrid models was found in the context of chemical kinetics and models of atoms (Justi and Gilbert, 2002b), and for describing phenomena in the context of genetics in six different countries (Gericke and Hagberg, 2010a; Gericke et al., 2012). Furthermore, the models presented in the textbooks can cause students' difficulties in understanding, as indicated by, for instance, Gericke and Hagberg (2010b) in the context of genetics. They found that the models presented in textbooks were correlated to the alternative conceptions held by students. Furthermore, they indicated that if these textbooks are used as a foundation for teaching, this correlation may persist. Teaching models are frequently used in chemistry textbooks, and studies have shown that these teaching models failed both to support the students' understanding of a certain aspect of content and of the meaning of a model (Justi and Gilbert, 2002b). Consequently, it is important that the teachers as well as the textbook writers are aware of both how the models are presented in textbooks and what teaching models they use might influence the students' understanding.
In this study, we focus on analyzing the models of chemical bonding presented in school textbooks based on the aforementioned research literature regarding students' difficulties in understanding.
Aim and research questions
The aim of this study is to investigate how chemical bonding models are expressed, that is, represented in different modes of representations (verbal, symbolic and visual mode) in school chemistry textbooks related to students' difficulties in understanding (concerns alternative conceptions and difficulties in understanding). The study was guided by the following research questions:• To what extent can representations of chemical bonding, in different chemistry textbooks, be identified that are relevant from the perspective of students' difficulties in understanding chemical bonding?
• In what ways the representations of models of chemical bonding might cause students to have difficulties in understanding?
Method
In this study, content analysis was the main approach to analyze chemistry textbooks at upper secondary level. Content analysis is an approach to study the presence of certain words or concepts within texts or sets of texts (Palmquist et al., 1997). In previous sections, we have presented earlier research results concerning students' conceptions and difficulties in understanding models in general and chemical bonding models, and furthermore, we applied these results from a literature review to analyze textbooks. The sources of textbooks, the analytical method, and the validity and reliability of our analyses are presented in the following sections.The textbooks
To answer research question 1 and 2, chapters concerning chemical bonding were analyzed in five chemistry textbooks published by different publishers for teaching at upper secondary level in Sweden (student's age from 16 to 19). The textbooks were chosen because they belong to the most widely used chemistry textbooks (all but one, TB4). The reason to include TB4 was that this study is part of a larger research project also including interviews with chemistry teachers and one of them was using this textbook.The school textbooks (indicated as TB1–TB5), and the related amounts (pages) of content regarding ionic, covalent, polar covalent and metallic bonding, respectively, are presented in Appendix 1.
Content analysis and analytical framework
The analytical framework (a total of eleven categories, Table 1) used in this study was based on the literature review of students' difficulties in understanding regarding models of chemical bonding as well as models in general and sources of these learning difficulties (e.g. Justi and Gilbert, 2000; Taber and Coll, 2002). The framework was developed from the data together with the research literature. For instance, category eleven, not addressed in research literature, was added because the data indicated that both which typical examples were used as well as how they were used might be a source of students' difficulties in understanding. The representations of chemical bonding models were, in categories one to nine, divided into three modes of representation: verbal, symbolic, and visual modes, according to Gilbert (2007).| The categories | The modes of representation— | ||
|---|---|---|---|
| Verbal mode | Symbolic mode | Visual mode | |
| a From “Gymnasiekemi A”, by S. Andersson, A. Sonesson, O. Svahn and A. Tullberg, 2007, p. 70. Reprinted with permission of Cicci Lorentzson (illustrator).b From “Modell och verklighet”, by H. Pilström et al., 2007, p. 147. Reprinted with permission of the publisher.c From “Kemiboken”, by H. Borén, A. Boström, M. Börner, M. Larsson, S. Lillieborg and B. Lindh, 2005, p. 92. Reprinted with permission of Per Werner Shulze (illustrator).d From “Kemiboken”, by H. Borén, A. Boström, M. Börner, M. Larsson, S. Lillieborg and B. Lindh, 2005, p. 91. Reprinted with permission of Per Werner Shulze (illustrator).e From “Gymnasiekemi A”, by S. Andersson, A. Sonesson, O. Svahn and A. Tullberg, 2007, p. 70. Reprinted with permission of Emma Adbåge (illustrator).f From “Kemi A: Tema & Teori”, by C. Engström, P. Backlund, R. Berger, and H. Grennberg , 2005, p. 140. Illustrator: T. Widlund. Reprinted with permission of the publisher.g From “Gymnasiekemi A”, by S. Andersson, A. Sonesson, O. Svahn and A. Tullberg, 2007, p. 57. Reprinted with permission of Per Werner Shulze (illustrator). | |||
| 1. Use of octet rule and focus on electronic configurations | ‘All ions formed have attained noble gas structure, i.e. they fulfil the octet rule’ (ionic bonding, TB4, p. 137) |
 |
 |
| 2. Reason for why bonding occurs a. Octet rule b. Energy changes |
‘The driving force for reactions is that atoms strive to react so that they get noble gas shell’ (TB1) |  |
 |
| 3. Chemical bond due to electrostatic forces | ‘In the crystal, ions bond to each other with ionic bonding, constituted of the attraction between positive and negative ions’ (TB2) | None |  |
| 4. Focus on separate atoms when representing chemical reactions | ‘Each sodium atom donates one electron and each chlorine atom accepts one electron’ (ionic bonding, TB4, p. 137) |
 |
 |
| 5. Anthropomorphism and chemical processes | ‘The atoms attain noble gas structure by commonly owning the electrons in the bond’ (TB4) | None |  |
| 6. Chemical bonding presented in terms of b. Electron sharing |
‘Ionic bonding: electrons are donated by one atom and accepted by another’ (electron transfer, ionic bonding, TB4, p. 140) ‘A covalent bond where the electrons are not shared equally between the bonded atoms is called polar covalent bonding’ (polar covalent bonding, TB1, p. 56) |
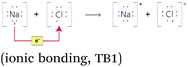  |
 |
| 7. Attribute from different historical models merged to hybrid models | Two electrons form a pair, communally for both atoms (electron sharing), and then surrounded by the same electron cloud (quantum mechanical model of atom, QMA) as a noble gas (octet rule, OF). For hydrogen, one can do good calculations of how the electrons behave (QMA), and then get a picture of the density of the electron cloud (molecule orbital theory, MO). (covalent bonding, TB5, p. 150) |
None | 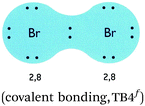 |
| 8. Bonded non-molecular materials presented as involving discrete molecules | ‘The ion pair Na+Cl− is the crystal's smallest ‘building element’. (ionic bonding, TB2, p. 57) |
2Na + Cl2 → 2Na+Cl− (ionic bonding, TB1) |
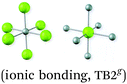 |
| 9. Explaining nature and purpose of models | ‘The models used here are strongly simplified, yet useful’ (TB4) |
None | None |
| 10. Order of introducing types of bonding | 1. Ionic bonding, 2. covalent bonding, 3. polar covalent bonding, and 4. metallic bonding (TB1, TB3 and TB4) |
||
| 11. Use of typical examples | Sodium chloride (ionic bonding, all textbooks) |
||
Table 1 shows the categories and related examples. It is worth mentioning that the representations can be found in more than one category: for instance the example of the visual mode of representation shown in Table 1, classified under category number four, ‘Focus on atoms’, is also classified under category number six, ‘Electron transfer or electron sharing’. Regarding category two, ‘reason for why bonding occurs’; three, ‘chemical bond due to electrostatic forces’; and nine, ‘explaining model's nature and purpose’, the headings of these categories are not by themselves a source of students' difficulties in understanding: actually lack of or inappropriate reason for why bonding occurs, not presenting chemical bonding as due to electrostatic forces and not explaining the model's nature and purpose might be sources. A textbook was coded as using representations according to each category to a ‘large extent’ if the type of representation occurred repeatedly in either one or several modes of representations, that is, in all modes of representations altogether. If representations occurred according to the specific category only occasionally, a textbook was coded as using the specific type of representations to a ‘small extent’. The process of conducting the content analysis was as follows: the first author conducted a brief analysis of different modes of representations (verbal, symbolic and visual mode) of chemical bonding models that were presented in the textbooks. Then, a first set of categories was developed from the data together with the research literature regarding students' learning difficulties. This first set of categories was applied to analyze the chapter of ionic bonding in TB1. The categories were then discussed by all authors till an agreement was reached. The revised version of the categories was applied iteratively by the first author to analyze the remaining types of bonding in TB1. Again, the other authors contributed with some critical excerpts till an agreement was reached. This revised set of categories (Table 1) was then applied to all the textbooks, for each type of bonding respectively. The detailed analysis of the textbooks was conducted using the following steps:
First, a grid was constructed with the set of categories and modes of representation, one separate grid for each type of bonding. Every analytical unit was coded with a combination of letter according to the category and mode of representation, and numbered, and the codes were marked in the textbook. Then, the analytical units with the related codes were, for the verbal mode of representations, copied into the grid for each textbook. The analytical units for the symbolic and visual modes of representations were described briefly in words and inserted with their codes in the grid. The analytical units in the verbal modes are in terms of a paragraph, and in the symbolic and visual mode in terms of the particular formula, graphs, etc.
Furthermore, the categories were, when useful, divided into sub-categories depending on common characteristics of the representations.
Besides, a compilation was produced for each type of bonding. Finally, the textbooks were compared with each other to identify similarities and differences between them.
Result and discussion
Firstly in this section there is a presentation and discussion of the results regarding research question one: the extent to which chemical bonding is presented relevant from the perspective of students' difficulties in understanding (i.e. according to the categories shown in Table 1). After that, examples of representations of chemical bonding models are presented and in what ways they can cause students' difficulties in understanding are discussed (research question two), in subsections according to the categories. The first three categories are merged into one sub section. Category eight and eleven are presented when relevant in connection to subsections of the other categories. Furthermore, the quotes from the textbooks were translated from Swedish to English by the first author and discussed with the second author till an agreement was reached.Extent to which chemical bonding is presented relevant from the perspective of students' difficulties in understanding
We found examples of all sources of students' difficulties in understanding in all textbooks with one exception (TB4, hybrid models). For instance, all textbooks repeatedly used the octet rule and focused on electronic configurations when chemical bonding was explained; the octet rule as a reason for bonding; anthropomorphism in the context of chemical processes; and representations in terms of electron sharing to present covalent bonding. Besides, chemical bonds were presented as to be formed due to electrostatic forces to a small extent or not at all (all textbooks). Furthermore, only three of the textbooks (TB3, TB4, TB5) explained the model's nature and purpose, and then to a small extent. An overview of the way and extent to which chemical bonding is presented in relation to the sources of students' conceptions and difficulties in understanding in the school textbooks is presented in Table 2. Regarding category ten and eleven, the extent is not relevant, that is, the order of introducing types of bonding could only be in one way, and the examples are only regarded relevant if used or not.| The categories used to identify representations that might be sources of learning difficulties | TB1 | TB2 | TB3 | TB4 | TB5 |
|---|---|---|---|---|---|
| 1. Use of octet rule and focus on electronic configurations | L | L | L | L | L |
| 2. Reason for why bonding occurs | |||||
| a. Octet rule | L | L | L | L | L |
| b. Energy changes | L | L | |||
| 3. Chemical bond due to electrostatic forces | |||||
| a. Ionic | S | S | S | S | S |
| b. Covalent and polar covalent | — | S | — | S | S |
| c. Metallic | — | S | — | S | S |
| 4. Focus on separate atoms when representing chemical reactions | L | L | S | L | L |
| 5. Anthropomorphism and chemical processes | L | L | L | L | L |
| 6. Chemical bonding presented in terms of | |||||
| a. Electron transfer | L | L | S | S | L |
| b. Electron sharing | L | L | L | L | L |
| 7. Attributes from different historical models merged to hybrid models | L | L | S | — | L |
| 8. Bonded non-molecular materials presented as involving discrete molecules | L | L | S | S | L |
| 9. Explaining the models' nature and/or purpose | — | — | S | S | S |
| 10. Order of introducing types of bonding | |||||
| a. Ionic, covalent, polar, metallic | X | X | X | ||
| b. Metallic, ionic, covalent, polar | X | ||||
| c. Ionic, covalent, polar, intermolecular forces, metallic | X | ||||
| 11. Use of typical examples | |||||
| a. Ionic bonding: NaCl | X | X | X | X | X |
| b. Covalent bonding: H2 | X | X | X | X | X |
| c. Polar covalent: HCl | X | X | X | — | X |
In what ways the representations of models of chemical bonding might cause students to have difficulties in understanding
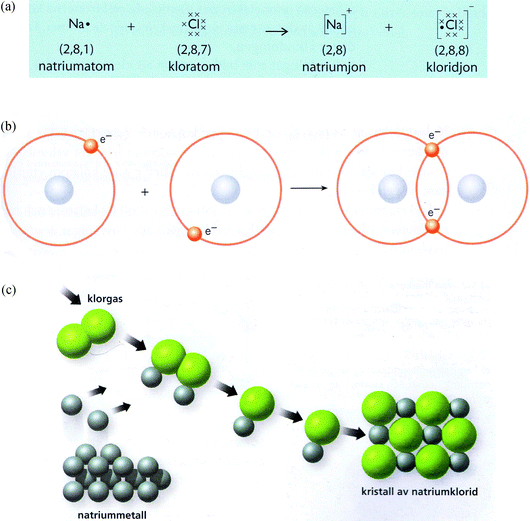 | ||
| Fig. 2 Representations of chemical bonding models expressing focus on electronic configuration and focus on atoms when representing chemical reactions. In terms of (a) Lewis dot symbols the formation of sodium and chloride ions, TB4 (From “Kemi A: Tema & Teori”, by Engström et al. (2005, p. 137). Illustrator: T. Widlund. Reprinted with permission of the publisher), (b) Bohr's atomic model, the formation of a hydrogen molecule, TB3 (From “Kemiboken”, by Borén et al. (2005, p. 99). Reprinted with permission of Cecilia Frank (illustrator)), (c) Representation of chemical bonding models that could be interpreted as ionic lattices contain molecules or ion-pairs seen as if they were molecules: the formation of sodium and chlorine ions where the reactants are shown as molecules and lattice structure (From “Gymnasiekemi A”, Andersson et al. (2000, p. 41). Reprinted with permission of Per Werner Shulze, illustrator). | ||
Except for the ‘octet rule’, the only other reason for bonding to occur, explained by three of the textbooks, was energy changes. However, this is only scarcely used and is not always explicit. These energy changes were in terms of (a) energy is released/required when a bond is formed/broken, or (b) molecules or ionic lattices (product) contain less energy than free atoms/ions (reactants). For instance, an example of energy changes related to chemical bonding in a verbal mode of representation: ‘when the molecule [hydrogen] is formed energy is released. The hydrogen molecule then contains less energy than free hydrogen atoms together’ (p. 47, TB1). There are also representations in symbolic and visual modes that present energy changes in conjunction to chemical bonding, as shown in Fig. 3 and Table 1, category 2. A majority of these examples concerns ionic or covalent/polar covalent bonding. For metallic bonding, however, there are only three representations (TB2) in total in all textbooks that in some way connect energy and chemical bonding.
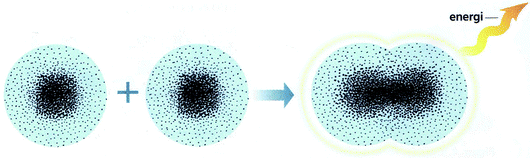 | ||
| Fig. 3 Representation of chemical bonding models connecting energy and chemical bonding in terms of: electron cloud, formation of a molecule of hydrogen molecule, TB1 (From “Gymnasiekemi A”, by Andersson et al. (2000, p. 47)). Reprinted with permission of Jan-Olof Sandgren, illustrator. | ||
Regarding chemical bonding in terms of electrostatic forces, three of the textbooks (TB1, TB3, TB5) introduce chemical bonding in general in terms of electrostatic forces. Either by saying that chemical bonding is an attraction between positive and negative charges, connected to energy, or a force that bonds atoms or the building block of matter together. The other two textbooks introduce chemical bonding in terms of atoms striving for a noble gas shell. However, when we look at how the different types of bonding, respectively, are presented in regard to electrostatic forces, there are some important differences. On the one hand, all textbooks expressed ionic bonding due to electrostatic forces in verbal modes of representation, even though they are presented mainly in terms of electron transfer. For instance, ‘because the ions have opposite charges, they are bonded to each other by electrostatic attractions’ (TB1, p. 43). All textbooks mentioned electrostatic attractions between the ions in the ionic lattice. However, ionic bonding is mainly introduced in terms of electron transfer.
On the other hand, we found only one non-verbal mode of representation in one textbook (TB3: Table 1, category 3, visual mode) that presented ionic bonding in terms of electrostatic attraction. Actually, this was the only example presenting chemical bonds in terms of electrostatic forces in total for all bonding.
In contrast to ionic bonding, none of the textbooks use the term electrostatic force or even attraction force to explain covalent and polar covalent bonding. Instead, the few representations that could be interpreted as bonding due to electrostatic forces were in terms of attractions or pulling between: the atoms' nucleus (covalent bonding); or the electrons and atoms, which actually are not charged (polar covalent bonding). Compared to the ionic lattice, no verbal representation was identified which presented the bonds in the giant covalent lattice as due to electrostatic forces. However, TB3 states that there are forces acting between the atoms in a molecule, but does not specify these forces as electrostatic or acting between charged particles as electrons and nucleus. Furthermore, TB1 says that hydrogen molecules do not attract each other as ions with opposite charges, although there must be some force of attraction between the molecules. But, nothing is said regarding the forces between the atoms in the molecule.
Three of the textbooks present metallic bonding in terms of attractions between the positive ‘metallic ions’ and the electron cloud or delocalized electrons. For instance, in verbal mode of representation: ‘The metal is held together by attraction between the positive ions and the delocalized valence electrons’ (TB5, p. 167). However, even though the other two textbooks also use the terms ‘metallic ions’ and/or ‘electron clouds’, they are not explicit regarding ions and the electron cloud acting reciprocally. For instance, ‘One or more of the valence electrons […] are free to move between the atoms. They constitute an electron cloud that belongs to the whole metallic crystal and give rise to the uniting forces in the crystal’ (TB1, p. 61). Yet, all textbooks use similar representations in visual mode as shown in Fig. 4. But, the term electrostatic attraction force was used by one textbook only (TB4). The term ‘electron cloud’ has the same meaning as the term ‘sea of electrons’ used in the scientific model of metallic bonding not based on quantum mechanics. In verbal mode of representation, the cloud is said to ‘be everywhere’ (TB1, p. 61), or the electrons are ‘flowing through the metal’ (TB2, p. 61). Furthermore, the movement of the electrons in the electron cloud (TB1, TB2, TB4), or that the cloud is mobile (TB5), is used to explain the conductivity of metals. For instance, ‘the delocalized electrons [in the electron cloud] can easily move around in the metal, and they are the cause of the conductivity of the metal’ (TB4, p. 142); or ‘the cloud is also mobile which causes the metal to conduct electric current and heat’ (TB5). Yet, TB3 do not explicitly explain the conductivity.
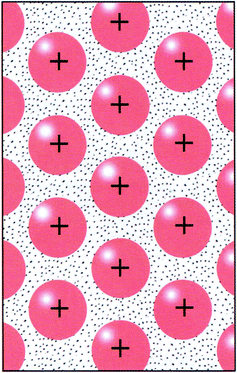 | ||
| Fig. 4 Visual mode of representation expressing positive metallic ions surrounded by electron cloud, TB5 (From “Modell och verklighet”, by Pilström et al. (2007, p. 166). Reprinted with permission of the publisher). | ||
We found that the octet rule and focus on electronic configuration is used to a large extent in all textbooks and express the octet rule as a reason for bonding. However, ‘the octet rule’ is a limited teaching model, over-generalised by the students, and a source for students to develop the octet framework (Taber and Coll, 2002). The octet framework might influence students' thoughts about bonding (i.e. students think that atoms want to have ‘octets’ or ‘full outer shell,’ and this is the reason for chemical processes to occur). Therefore, Taber and Coll suggest that bonding should be explained in terms of electrical forces. This would be an ‘authentic teaching model […] at an optimal level of simplification’ (p. 218), which can facilitate the students' understanding of bond polarity, electronegativity and inter-molecular bonding, and to prepare for more sophisticated chemical bonding models based on quantum mechanics at university chemistry level. Furthermore, if there is a lack of discussion on why chemical reactions occur, it leads to an ‘explanatory vacuum’ (p. 217). In addition, the presence of a feasible alternative explanation, (i.e. the octet rule), can contribute to the assumption that the octet rule is the reason for bond formation. Even though we identified representations in terms of electrostatic forces in the textbooks (e.g. in all textbooks regarding ionic bonding), these representations were mainly in regard to ionic bonding and then more as a constitution of the bonds, not as a reason. Also, they were presented alongside or even in the context of bonds formed to achieve a noble gas shell. Besides, ionic bonding due to electrostatic forces is presented in verbal mode of representation only with one exception, which could be an additional source to students' preference in identifying ionic bonding with electron transfer instead of electrostatic forces.
Furthermore, regarding metallic bonding and electrostatic forces, de Posada (1999) reported difficulties of students in explaining the strong attractive force between components of metals. These difficulties are suggested to derive from insufficiently explanations in textbooks and by teachers (de Posada, 1999). Similarly, we found that only three of the textbooks in the present study explicitly represent metallic bonding in terms of cations and the electron cloud act reciprocally. Besides, in line with what is reported by de Posada (1999), all but one (TB1) of the textbooks mix the words ‘atom’ and ‘cation’ (or ‘metal ion’ or ‘positive ion’) when they represent metallic bonding. Thereby, it might be difficult for the students to understand that there are electrostatic forces between the electron cloud and the positive ‘metallic ions’, because atoms are not charged.
The term ‘sea of electrons’ (or, as used in the Swedish textbooks, ‘electron cloud’), however, is reported to be difficult for students to understand (Taber, 2001): students could be so influenced by this metaphor that they conceptualize this sea as a vast excess of electrons, which actually would be charged and unstable. Hence, the visual mode of representation shown in Fig. 4, and verbal mode of representation as ‘the cloud is said to be everywhere’, or ‘electrons are flowing through the metal’, could give the impression of an excess of electrons. Finally, in accordance to de Posada's findings of presentation of metallic bonding in textbooks (1999), we found that the textbooks do not clearly explain the relationship between electric current, electrons and conductivity of metals, a relationship which students find difficult to understand (de Posada, 1997).
Focus on separate atoms when representing chemical reactions
Ionic, covalent and polar covalent bonding are, in all textbooks, introduced by presenting a hypothetical fictional account of the origin of the bonding, that is, for ionic bonding by presenting the formation of ions constituting the ionic compound, and for covalent and polar covalent bonding the formation of a molecule. A focus on separate atoms when representing these chemical reactions related to bond formation exists in all textbooks, especially to explain ionic bonding since all textbooks present ionic bonding in terms of electron transfer between atoms (i.e. the formation of ions). In other words, they use representations in terms of interactions between discrete atoms, when the reactants actually are composed of molecules or lattice structure. The focus on atoms is exemplified by, in verbal mode of representations: ‘The chemical reaction between sodium and chlorine means that one sodium atom donates one electron that is accepted by one chlorine atom’ (TB5, p. 35); and in symbolic and visual mode of representation as shown in Fig. 2a and b and Table 1, category 1, 2, and 4. In contrast, a visual mode of representation that actually does not present interactions between discrete atoms and consequently does not focus on atoms is found in TB1 (Fig. 2c) and TB2: in terms of a schematic description of formation of a sodium chloride lattice from molecules and lattice structure.The focus on separate atoms regarding chemical reactions can be seen as a source for students to develop the octet framework, because the fecundity of the octet rule depends on the students' conception that everything is derived from and comprised of atoms (Taber and Coll, 2002). Here we have found that there is a focus on separate atoms when representing chemical reactions related to bond formation to a large extent in all but one textbook (TB3).
Even if metals are elements and not compounds, most metals do exist as compounds in humans under normal conditions. Therefore, they need to be prepared by chemical processes. Moreover, there also exist metal bonds in metal alloys. But metallic bonding is not introduced by presenting a hypothetical fictional account of the origin of the bonding in any of the textbooks. This can be compared to the introduction of covalent bonding, where for instance hydrogen and chlorine are used as typical examples. These are also elements but yet presented by the formation of molecules from separate atoms. If the bonding in, for instance, sodium chloride and hydrogen needs to be explained by the formation of the particles (i.e. ions and molecules), one can argue that so does the bonding in, for instance, aluminium, that needs to be prepared from aluminium oxide. As discussed in the section below, however, the representation of ionic bonding in terms of electron transfer between atoms has been strongly criticised because it could lead to several alternative conceptions. Therefore, one can raise the question if the bonding types should be introduced by presenting a hypothetical fictional account of the origin of the bonding or not.
Anthropomorphism and chemical processes
Anthropomorphism for explaining chemical processes exists in all textbooks, numerously and in a generous variation, mainly to present ionic, covalent and polar covalent bonding. In terms of social situations, e.g. the atoms are said to donate, accept, lose, or get electrons (ionic bonding), share, share equal or unequal, donate, accept, get or have access to, pass over, agree, commonly own, pull on, attract, or contribute with electrons or have no equality (covalent and polar covalent bonding); electrons move over, be overdrawn, ripped away, (ionic bonding), belong equal or unequal to both atoms, pull the nucleus, attend or participate in bonds, stay in between the nucleus, and be drawn towards atoms (covalent or polar covalent bonding); ions belong to or be free from each other, the electron cloud or electron pair is commonly or pulling the nucleus; molecules behave. In terms of psychological states, the atoms are said to strive for a noble gas shell (bonding in general, ionic, covalent bonding), willingly or easily donate (ionic, covalent bonding), and need or must have electrons.Regarding metallic bonding, however, the anthropomorphic descriptions are mainly in terms of the electron cloud or atoms and social situations, but they are neither as common nor as generous in variation as for ionic, covalent and polar covalent bonding. The electron cloud is said to be commonly, belong to the whole crystal, the atoms donate electrons, have neighbours, and electrons are shared.
Several of these anthropomorphic descriptions are consistent with the language used by students according to Taber and Coll (2002). We described in the background section when anthropomorphic explanations could become an impediment to further learning. In regard to these arguments we think that the authors of school textbooks may use the anthropomorphic explanation less frequently and point out that these explanations provide a way of starting to think about chemical bonding; that is, it is “a bit like this”.
Chemical bonding presented in terms of electron transfer or electron sharing
As mentioned above, all the textbooks introduce ionic bonding in terms of electron transfer between atoms to form ions. For instance, TB4 says that: ‘We can use a dot/cross diagram to show how the atoms are bonded to each other’, (p. 137), when actually it represents the formation of ions in term of electron transfer (Fig. 2a), not the ionic bonding in itself.Furthermore, all typical examples used in the section concerning ionic bonding, in all textbooks, represent ionic bonding as the result of electron transfer. In symbolic mode there are numerous examples of such representations, e.g. as shown in Fig. 2a and Table 1, category 4.
Similar to ionic bonding, representations of metallic bonding in verbal mode in terms of electron transfer were identified. For instance, ‘each atom donates electrons from the outer shell so that the inner part of the atoms form positive ions’ (TB4, p. 142); and ‘electrons from one atom can go over to atoms that lie next to another’ (TB3, p. 111).
As mentioned, we found numerous representations of ionic bonding in terms of electron transfer. This representation has been strongly criticised because it could lead to: the conception that an ionic compound contains molecules (Taber and Coll, 2002; Taber, 2003b); ionic bonds only exist between ions that had transferred electrons (Taber and Coll, 2002; Taber, 2003b); and students identifying ionic bonding with electron transfer instead of electrostatic forces (Taber and Coll, 2002). Furthermore, we found additional representations which might be interpreted as an ionic lattice contains molecules or ion-pairs seen as if they were molecules. For instance: the ion pair Na+Cl− is the smallest “building element of the crystal” (TB2, p. 57); the representations in Table 1, category 8, symbolic and visual modes; the schematic description of the formation of a sodium chloride lattice, where the sodium atoms are attached to the chlorine atoms, forming pairs of ions attached to each other (Fig. 2c). In the last example, the balls representing the atoms and ions have the same size before and after the reaction. This could be a source of the conception that atoms are present in ionic compounds (Othman et al., 2008). Yet, only one textbook (TB3) pointed out that the chemical formula for sodium chloride, NaCl, does not imply that there are ion-pairs, and another explicitly says that there are no molecules of sodium chloride in the lattice (TB5). Although the representation shown in Table 1, category eight, visual mode, might be a way to show that ions in the lattice are surrounded by several ions in all directions and no molecules are present, in our opinion, the result might be the opposite.
In addition, covalent and polar covalent bonds are presented with numerous representations in terms of electron sharing, i.e. as electrons shared by two atoms in a molecule. In verbal modes, the representations were in terms of
• the electron pair is shared, shared equally or unequally
• the electrons or electron pair are communal (all textbooks)
• the binding electron pair
• the electron pair bonds the atoms together
• the bond is constituted by an electron pair
There are also representations where the term ‘electron clouds’ is used alongside the term ‘electron pair’ in the context of sharing.
Besides, we also identified representations in symbolic mode of electron sharing, in terms of Lewis structure and Bohr's atomic model (Fig. 5a and Table 1, category 1). But, the ball and stick model is mainly used to represent the giant covalent lattice as graphite and diamante (all but TB4, Fig. 5b), and fullerenes, and regarding separate molecules in the context of explaining the shape of molecules and dipoles. However, TB5 is the only textbook that repeatedly uses space-filling models to represent molecules with covalent bonding (Fig. 5c).
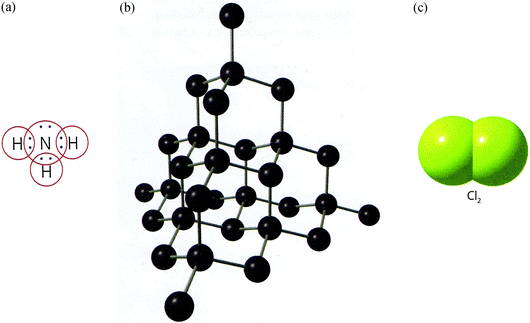 | ||
| Fig. 5 Representations of chemical bonding models that show (a) Lewis structure with dots and circles around the shared electron pair, TB1. (b) Ball and stick model of the giant covalent lattice of diamante TB2 (From “Gymnasiekemi A”, by Andersson et al. (2007, p. 73). Reprinted with permission of Per Werner Shulze, illustrator). (c) Space-filling model of a molecule of chlorine, TB5 (From “Modell och verklighet”, by Pilström et al. (2007, p. 151). Reprinted with permission of the publisher). | ||
For metallic bonding, similar to explanations on covalent bonding, the expression communal electron cloud is used. The electron cloud is said to hold the crystals together or give rise to the uniting forces (TB1, TB2), in the same way that the atoms in a molecule are said to be held together. Furthermore, when the bonding between metallic atoms in metals is explained, the electrons are said to be shared like in covalent bonds, ‘but now by all the atoms in the metal and not only by two atoms’ (TB4, p. 142),
However, a common conception among students is that the shared electron pair in itself is the bond and the electron pair holds the atoms together because they then receive a noble gas shell (Taber and Coll, 2002). The conception could be reinforced by the formation of bonds presented as formed in order to achieve a noble gas shell, and bonds not presented as due to electrostatic forces. We think that especially expressions such as (i) ‘binding electron pair’, (ii) ‘the electron pair bonds the atoms together’ and (iii) ‘the bond is constituted by an electron pair’, could reinforce the conception that the electron pair is the bond. Besides, the merging of electron cloud and electron pair could be an additional source of confusion for the students. Furthermore, the ubiquitous use of the ball and stick model could lead to the conception that bonds are very small strings or lengths of strings (Butts and Smith, 1987; Taber and Coll, 2002). Space-filling models do not focus on the shared electron pair, but TB5 is the only textbook that repeatedly uses space-filling models.
In addition, we found some ambiguities when polar covalent bonding was explained. All textbooks present examples of molecules with polar covalent bonding in the context of covalent bonding, before polar covalent bonding is defined. Polar covalent bonding is then defined, without explicitly saying that the molecules of chemical compounds presented in the previous section are actually examples of polar covalent bonding. Furthermore, one of the textbooks (TB2) uses the term ‘covalent’ instead of ‘polar covalent’ even after the concept of polar covalent bonds is defined. We also found that the concept of polar molecules was explained in conjunction to and in the context of polar covalent bonding (all but TB4), e.g. a polar molecule was presented below a heading marked ‘polar covalent bonding’. As a typical example used in conjunction to defining polar covalent bonding all but one textbook (TB4) used hydrogen chloride.
Moreover, the textbooks are not always clear with regard to the fact that polar covalent bonding in the molecule is not necessarily equal to the polar molecule. Further, the students are supposed to make the conclusion themselves that the molecules of chemical compounds presented in the section of covalent bonding actually are examples of polar covalent bonding. We think that the textbooks do not clearly distinguish between covalent and polar covalent bonds and also are unclear about how polar covalent bonds are related to polar molecules. This might lead to further confusion about these concepts.
Regarding the conception that there is covalent or ionic bonds in metals (Taber, 2001, 2003a), or no proper chemical bond (Taber, 2001), Taber and Coll (2002) suggest that this depends on the presentation of ionic and covalent bonds as a dichotomy. This dichotomy emerges in all the textbooks by frequent use of representations that assume bonds to be formed either by transfer of electrons (ionic bond) or sharing electrons (covalent bonds) in order to obtain a noble gas shell, as presented in this sub-section and previous sub-sections. Even if there are some representations in terms of transfer and sharing of electrons regarding metallic bonds, as presented above, the textbooks do not use the octet rule and/or focus on electronic configurations at all in the context of metallic bonding: these actions seem to be not done in order to obtain a noble gas shell, which might confuse the students.
Attributes from different historical models merged to hybrid models
We identified hybrid models in all but one textbook (TB4) in the context of covalent and polar covalent bonding. Attributes from models based on quantum mechanics are merged with the model for covalent and polar covalent bonding not based on quantum mechanics, i.e. in terms of electron sharing. None of the textbooks point out the fact that different models can be used to explain chemical bonding. Besides, they do not mention what specific model the attributes derive from. The hybrid models we identified are expressed in verbal modes of representation only, or in a combination of verbal and visual or symbolic modes. The following attributes identified from models based on quantum mechanics were found:• probability of the electrons to be located between the nuclei (valence bond theory, VB).
• density of electrons between the atoms (molecule orbital theory, MO).
• electrons of the atoms described as electron cloud (quantum-mechanical model of the atom, QMA).
• calculations of the electron movements (QMA).
• communal electron cloud for the molecule (MO).
• atomic orbital overlap (VB)
We here give one example which describes how the different attributes are combined to form a hybrid model, found in TB2 (p. 70):
“The [atoms'] electron clouds [QMA] begin to overlap each other [VB] and form a communal cloud surrounding both the nucleus [MO]. The electrons belong just as much to both the atoms [electron sharing] [...] the probability to find the electrons is highest around the nucleus and in the area in between them [VB]. There is the communal electron cloud especially dense [MO] [...]. The two electrons in the communal cloud [QMA] pull the nucleus toward each other and in that way bond the atoms together into a hydrogen molecule. The electrons constitute a bonding electron pair [...] and are shared equally [electron sharing]”.
In addition, we also identified separated verbal mode of representations that could be seen as derived from the probability of the electrons being located between the nuclei (VB), for example: ‘the electrons stay more often between the atom nuclei, where they bond the atoms together’ (TB3, p. 98).
Moreover, in the context of metallic bonding we identified attributes in verbal mode from the band theory (an extension of the MO theory), in terms of overlap between the atoms' orbital: ‘The atoms are so closed packed that their valence electrons partly overlap each other’ (TB3, p. 111). Besides, there is a simplification (or alternative conception) in this statement: it is the atoms' orbitals which overlap each other; valence electrons do not overlap. In the context of comparing metallic, ionic and covalent bonding, we also identified the statement that metallic bonding is said to be ‘an extreme form of covalent bonding’: there is no sharp limit between metallic bonding and covalent bonding. One can actually see metallic bonding as an extreme form of covalent bonding. In covalent bonding, only a small number of bonding electrons are commonly for two atoms, but, in metallic bonding, a large number of electrons are delocalized (i.e. commonly for) all atoms in the whole crystal’ (TB2, p. 85). This statement might originate from the fact that band theory is an extension of the MO theory, a model to explain covalent bonding.
Furthermore, hybrid models in terms of different attributes used in a combination of verbal and visual mode were also identified. For instance, TB1 and TB2 say in verbal mode that the electron clouds (QMA) move in to each other (VB) and that the atoms are sharing two or more electron pairs (electron sharing). This is done in conjunction with the visual mode of representation in terms of a hydrogen molecule with the electron cloud (QMA) represented in terms of coloured circles that intersect (Fig. 6a). Furthermore, when describing the structure of graphite, the concept of the electron cloud (QMA) is used in the verbal mode of representation. However, in the visual mode of representation, the structure is then presented in terms of a ball and stick model (Fig. 6b), that is, as the sharing of electrons, without showing any electron clouds.
 | ||
| Fig. 6 Visual mode of representation, showing (a) a molecule of hydrogen in an electron cloud that intersects, TB1 (b) structure of graphite, ball and stick model, TB1 (From “Gymnasiekemi A”, Andersson et al. (2000, p. 56 (a) and p. 49 (b)). Reprinted with permission of the publisher). | ||
As mentioned in previous sections, the use of hybrid models can cause difficulties in teaching and learning, and may be a source of confusion for students (Justi and Gilbert, 2000; Gericke and Hagberg, 2007). Here we found hybrid models in all but one textbook (TB4), and to a large extent in three of the textbooks. This is also in line with results from analysis of textbooks in the context of models of genetics; a pronounced use of hybrid models was found (Gericke and Hagberg, 2010a; Gericke et al., 2012).
Explaining the models' nature and/or purpose
The nature of a model in the context of chemical bonding is explicitly mentioned in three of the textbooks (TB3, TB4, TB5), although only once. For instance, ‘The models used here are strongly simplified, but yet useful in many simple cases. To explain more complicated cases, the models need to be refined’ (TB4, p. 143). And as stated in TB3, ‘Models are always incorrect, but they are often useful’. (TB3, p. 93).Furthermore, TB5 uses three different models in visual mode of representation to explain the bonding in hydrogen molecules, and states that one of the models ‘best corresponds to reality’ (p. 159). Besides, the fact that models might be changed and revised during history is shown in TB5: an example of how chemists viewed ionic and covalent bonding in the beginning of the twentieth century. In addition, several implicit verbal modes of representations expressing the nature of models are found: statements such as the atoms ‘can be thought to be’ and phrases about there being different ‘ways’ or models to describe something. In these situations, the ‘ways’ or models refer to symbolic or visual modes of representation, with one exception (TB4).
It is important for the students to have knowledge of and recognize the models’ functions and limitations as well as the fact that a concept can be explained by several models (Boulter and Gilbert, 2000; Gericke and Hagberg, 2007; Drechsler and Van Driel, 2008). However, we mainly found the latter in the textbooks: the models' nature is only rarely discussed, and the purpose is not discussed at all, in correspondence to what is found in research literature (Grosslight et al., 1991). Furthermore, we find the nature of models as described in TB3, ‘models are always incorrect’, as too simplistic. Finally, the models are often presented in the textbooks as if they are proven facts rather than entities created to highlight some aspects of theories, which also correspond to research literature (Treagust et al. 2002).
Order of introducing types of bonding
The order of introducing types of bonding in the school textbook was:• ionic bond, covalent bond, polar covalent bond, and metallic bond (TB1, TB3, TB4, UB2, UB3)
• metallic bond, ionic bond, covalent bond, polar covalent bond, (TB2)
• ionic bond, covalent bond, polar covalent bond, inter-molecular forces, metallic bond (TB5)
Teaching covalent bonding before ionic bonding is a common practice, which could lead to students seeing an ionic lattice as containing molecules (Taber and Coll, 2002). To avoid the students applying the ‘molecule presence’ to all structures, Taber and Coll suggest teaching metallic bonding first, followed by ionic bonding, and covalent bonding last. Besides, in the context of covalent bonding, they suggest to start with giant covalent lattices before discrete covalent molecules. On the one hand, we found that none of the school textbooks presented giant covalent lattices before discrete covalent molecules, and TB2 is the only one that presents metallic bonding first. On the other hand, we found that none of the school textbooks teach covalent and polar covalent bonding before ionic bonding.
Conclusions and implications
In our study we have shown that the school textbooks use representations of chemical bonding models in such ways that, according to research known for several years, might cause students to have alternative conceptions and difficulties in understanding chemical bonding. Hence, our results indicate that textbooks might cause students’ difficulties in understanding. Furthermore, students can have problems in reconstructing the scientific meaning of the models when reading their textbooks, which is in line with recent research findings in the context of genetics (Gericke and Hagberg, 2010b; Gericke et al., 2013). We identified representations of all the sources used as categories in the analysis, in all school textbooks (except for one category in one textbook, see Table 2). Clearly, there seems to be a gap between research on students' difficulties in understanding and textbook writers. To improve the students' understanding of chemical bonding, we suggest that the presentation of chemical bonding in textbooks should be changed with consideration of the reported research results of students' alternative conceptions and difficulties in understanding, as discussed in previous sections. Each type of bonding has different models and related problems in understanding. Yet, there are some suggested changes common for all types. To start with, to avoid the students applying the ‘molecule presence’ to all structures and developing the octet framework, we suggest that the order of introducing the types of bonding should be changed according to the proposal by Taber and Coll (2002): teaching metallic bonding first, followed by ionic bonding, and covalent bonding last, and in the context of covalent bonding to start with giant covalent lattices before discrete covalent molecules. In addition to this proposal, to continue the ‘lattice-way’ and to make a logical bridge to discrete molecules, we might suggest that after giant covalent lattices, lattices of compounds consisting of discrete molecules in solid state (e.g. solid of water) can be introduced. Also, emphasize that there are electrostatic forces both between the discrete molecules and between the atoms in the molecules. However, before explaining electrostatic forces between discrete molecules the bonds between atoms in the molecules and the concepts polar and non-polar molecules need to be explained. Moreover, even if substances have different properties that can be explained by the different types of bonding, we think it is important to emphasize also what is common for all types of bonding; forces hold the particles, which are constituting the substances, together, and then we suggest to use the teaching model proposed by Taber and Coll (2002), based upon the effect of electrostatic forces, for all types of bonding coherently. This can be an authentic teaching model at an optimal level of simplification that provides a ground for students for more sophisticated chemical bonding models based on quantum mechanics at university level (Taber and Coll, 2002). Moreover, the use of octet rule or focus on electronic configuration can then be reduced. Furthermore, by explaining chemical bonds due to electrostatic forces coherently, the students will be better prepared to understand, for instance, intermolecular forces, electronegativity and the polarity of a bond (Taber and Coll, 2002).Moreover, one issue we want to point out is if the bonding types should be introduced by presenting a hypothetical fictional account of the origin of the bonding or not. As presented and discussed in previous sections, this is not consequently done in the textbooks: ionic, covalent and polar covalent bonding are explained by the formation of the particles (i.e. ions and molecules). However, metallic bonding, although metals often need to be prepared by chemical processes and reactions, is not introduced by presenting a hypothetical fictional account of the origin of the bonding. The fact that bonds are broken and formed during chemical reactions, and explicit discussion about the reason for chemical reactions and thereby for bonding, are important and are examples of how the development of the octet framework by students could be avoided (Taber and Coll, 2002). But does this imply that bonding need to be introduced by presenting the origin of the bond? We think this issue needs further discussion and investigation. One strategy could be to introduce each bonding type by focusing on explaining the existing bonding and not to introduce by the formation of particles participating in the bond (e.g. ions). Furthermore, to use physical properties as the starting point, because one of the purposes of a model is to explain observed phenomena (Gilbert et al., 1998) as, for instance, physical properties. Actually, metallic bonding, in all textbooks, is introduced in this way.
To create teaching models at an optimal level of simplification, several models and/or attributes from several models can be used. But, in order to avoid hybrid models that can cause difficulties in teaching and learning, and may be a source of confusion for students (Justi and Gilbert, 2000; Gericke and Hagberg, 2007), the textbooks should then: be clear about the fact that several models are used and/or the origin of the used attributes; how these models differ from each other; and the limitations of the models. In connection to this topic, we suggest that the textbooks more explicitly and consequently explain the models’ nature and/or purpose: it is considered important for the students to have knowledge of and recognize the models' functions and limitations as well as the fact that a concept can be explained by several models (Boulter and Gilbert, 2000; Gericke and Hagberg, 2007; Drechsler and Van Driel, 2008).
Regarding changes specific for ionic bonding, in order to avoid the students to identify ionic bonding with electron transfer and related alternative conceptions, we suggest to avoid the introduction of ionic bonding in terms of transfer of electrons. Furthermore, if formation of ionic compounds from its elements is presented, representations where the reactants are shown as molecules and lattice structure (similar to Fig. 2c), which do not focus on atoms, are suggested. In addition to typical examples such as ionic compounds formed from its element (which involves the step of formation of ions where electrons are transferred), we propose to also use ionic compounds formed by precipitation when aqueous solutions of salts are mixed (which do not involve transfer of electrons). Moreover, representations in visual mode of the ionic lattice expressing the electrostatic forces between the ions, in more than one direction, should be added to the verbal modes which express this interaction. Finally, anthropomorphic explanations are suggested to be used less frequently and not uncritically, to prevent them to shift from standing-in to take the place of the explanation (Taber and Watts, 1996). This is a suggestion also relevant for all types of bonding.
Regarding changes specific for covalent and polar covalent bonding, we suggest the textbooks to be clearer regarding how polar covalent bonds are related to polar molecules: concepts which are reported as unclear to students (Harrison and Treagust, 1996; Peterson et al., 1989). Furthermore, to clearly distinguish between pure covalent and polar covalent bonds and typical examples of these types of bonds.
Moreover, ionic and covalent bonds presented as a dichotomy can be avoided if chemical bonding is presented as suggested above regarding bonding in general. This dichotomy is a reason suggested for students to see polar covalent bonds as a characteristic of the covalent bond instead of something in between ionic and covalent bonds (Taber and Coll, 2002). Furthermore, we suggest the teaching model of covalent and covalent bonds as sharing of electrons between two atoms to be replaced, as presented above, by the electrostatic forces between the nuclei of both atoms and the electrons between them. Hence, the students' conception that the shared electron pair constitute the bond, and hold the atoms together because they then obtain octets of electrons can be avoided (Taber and Watts, 2000; Coll and Treagust, 2002). Furthermore, we suggest using space-filling models, which do not focus on the shared electron pair, in addition to ball and stick model and Lewis structure.
Regarding changes specific for metallic bonding, the textbooks should be more clear and explicit when they explain the physical properties of metals (for instance electrical conductivity), in order to avoid the reported students ‘difficulties in understanding this topics’ (de Posada, 1997). Furthermore, the stability of the metallic lattice needs to be more explained. In addition, we also suggest the textbooks to avoid the mixing of the word ‘atoms’ and ‘metallic ions’ when they present metallic bonding. Hence, the understanding of the electrostatic forces between the electron cloud and the positive ‘metallic ions’ might be improved. Furthermore, if ionic and covalent bonds are not presented as a dichotomy, the conception that there is covalent or ionic bonds in metals might be prevented. Finally, none of the textbooks use the visual mode of representation ‘corpuscular electron model’ in addition to the electron sea model (Fig. 4). Modern modeling research emphasizes the use of multiple models (Grosslight et al., 1991; Harrison and Treagust, 1996), so both these visual modes might be used. But according to de Posada (1999), in that case it is important to accompany these representations by accurate and explicit comments.
It should be mentioned that an altered framework also has been suggested by Nahum et al. (2008): the ‘bottom-up framework’. In this framework, chemical bonding is introduced as a continuum of related concepts instead of different types of bonding, with an emphasis on electrostatic interactions, stability and focus on the nature of the chemical bond. We think that it is relevant that authors and teachers become aware of the importance of how the models are presented, and get knowledge of which representations might influence students' understanding negatively. Moreover, because representations that might cause students' difficulties in understanding obviously exist in the textbooks, and to a large extent, we argue that there is a need for filling in the gap between research and textbook writers. Hence, scientific research can improve the development of new and better textbooks. As we show in this study, this gap still exists although these research findings have been known for several years. We think it is important to solve and raise the question how this gap will be filled in, and who is responsible for this. Finally, we consider it important for teachers to review the textbooks critically. This should be of importance not only for the topic of chemical bonding, but for all science education. As pointed out by de Posada (1999), the teachers are the one who decide which textbook to use.
Future research should be done to evaluate the altered frameworks mentioned above and the suggested changes in the representation of chemical bonding. Furthermore, the analytical framework used in this study might be used to analyze chemical literature used at university level, to investigate if representations that might cause students' difficulties in understanding also exist at this level. Also, a follow-up study will focus on how teachers are presenting chemical bonding and the influence of textbooks on their presentation.
List of school textbooks and the related amounts (pages) of content
| Label | School textbook | Amounts of pages |
|---|---|---|
| T1 | Andersson et al., (2000), Gymnasiekemi A, 2nd edn, Stockholm: Liber | 23 |
| T2 | Andersson et al., (2007), Gymnasiekemi A, 3rd edn, Stockholm: Liber | 37 |
| T3 | Borén et al., (2005), Kemiboken, 3rd edn, Stockholm: Liber | 23 |
| T4 | Engström et al., (2005), Kemi A: Tema & teori, 2nd edn, Stockholm: Bonnier utbildning | 12 |
| T5 | Pilström et al., (2007), Modell och verklighet, Stockholm: Natur och kultur | 30 |
References
- Andersson S., Sonesson A., Stålhandske B. and Tullberg A., (2000), Gymnasiekemi A, 2nd edn, Stockholm: Liber.
- Andersson S., Sonesson A., Svahn O. and Tullberg A., (2007), Gymnasiekemi A, 3rd edn, Stockholm: Liber.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Boo H. K., (1998), Students' understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35(5), 569–81.
- Borén H., Boström A., Börner M., Larsson M., Lillieborg S. and Lindh B., (2005), Kemiboken, 3rd edn, Stockholm: Liber.
- Boulter C. and Gilbert J. K., (2000), Challenges and opportunities of developing models in science education, in Gilbert J. K. and Boulter C. (ed.), Developing models in science education, Dordrecht: Kluwer Academic Publishers, pp. 343–362.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17(1), 192–201.
- Coll R. K. and Treagust D. F., (2002), Exploring tertiary students' understanding of covalent bonding, Research in Science and Technological Education, 20(2), 241–267.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding, J. Res. Sci. Teach., 40(5), 464–486.
- de Posada J. M., (1997), Conceptions of high school students concerning the internal structure of metals and their electric conduction: structure and evolution, Sci. Educ., 81(4), 445–467.
- de Posada J. M., (1999), The presentation of metallic bonding in high school science textbooks during three decades: science educational reforms and substantive changes of tendencies, Sci. Educ., 83(4), 423–447.
- Drechsler M. and Schmidt H. J., (2005), Textbooks' and teachers' understanding of acid–base models used in chemistry teaching, Chem. Educ. Res. Pract., 6(1), 19–35.
- Drechsler M. and Van Driel J., (2008), Experienced teachers' pedagogical content knowledge of teaching acid–base chemistry, Res. Sci. Educ., 38(5), 611–631.
- Edling A., (2006), Abstraction and authority in textbooks: the textual paths towards specialized language, Dissertation, Uppsala: Acta Universitatis Upsaliensis.
- Ekvall U., (2001), Den styrande läroboken, in B. Melander and B. Olsson (ed.), Verklighetens texter: Sjutton fallstudier, Lund: Studentlitteratur, pp. 43–80.
- Engström C., Backlund P., Berger R. and Grennberg H., (2005), Kemi A: Tema & Teori, 2nd edn, Stockholm: Bonnier utbildning.
- Garnett P. J., and Treagust D. F., (1992), Conceptual difficulties experienced by senior school students of electrochemistry: electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29, 121–142.
- Gericke N. M. and Hagberg M., (2007), Definition of historical models of gene function and their relation to students' understanding of genetics, Sci. Educ., 16(7/8), 849–881.
- Gericke N. M. and Hagberg M., (2010a), Conceptual incoherence as a result of the use of multiple historical models in school textbooks, Res. Sci. Educ., 40(4), 605–623.
- Gericke N. M. and Hagberg M., (2010b), Conceptual variation in the depiction of gene function in upper secondary school textbooks, Sci. Educ., 19(10), 963–994.
- Gericke N. M., Hagberg M., Santos V. C., Joaquim L. M. and El-Hani C. N., (2012), Conceptual variation or incoherence? Textbook discourse on genes in six countries, Sci. Educ., advance online publication, DOI:10.1007/s11191-012-9499-8.
- Gericke N., Hagberg M. and Jorde D., (2013), Upper secondary students' understanding of the use of multiple models in biology textbooks – the importance of conceptual variation and incommensurability, Res. Sci. Educ., 43(2), 755–780.
- Gilbert J. K., (2007), Visualization: a metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in science education, Dordrecht: Springer, pp. 9–27.
- Gilbert J. K., Boulter C. and Elmer R., (2000), Positioning models in science education and in design and technology education, Developing models in science education, Dordrecht: Kluwer Academic Publishers, pp. 3–18.
- Gilbert J. K., Boulter C. and Rutherford M., (1998), Models in explanations, part 1: horses for courses? Int. J. Sci. Educ., 20(1), 83–97.
- Grosslight L., Unger C., Jay E. and Smith C. L., (1991), Understanding models and their use in science: conceptions of middle and high school students and experts, J. Res. Sci. Teach., 28(9), 799–822.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Ingham A. and Gilbert J. K., (1991), The use of analog models by students of chemistry at higher education level, Int. J. Sci. Educ., 13(2), 193–202.
- Justi R. S. and Gilbert J. K., (2000), History and philosophy of science through models: some challenges in the case of “the atom”, Int. J. Sci. Educ., 22(9), 993–1009.
- Justi R. S. and Gilbert J. K., (2002a), Modelling, teachers' views on the nature of modelling, and implications for the education of modellers, Int. J. Sci. Educ., 24(4), 369–387.
- Justi R. S. and Gilbert J. K., (2002b), Models and modelling in chemical education, in Gilbert J., De Jong O., Justi R., Treagust D. and Van Driel J. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer, pp. 213–234.
- Mikk J., (2000), Textbook: research and writing, Frankfurt am Main: Peter Lang GmbH.
- Nahum T. L., Mamlok-Naaman R. and Hofstein A., (2008), A new “bottom-up” framework for teaching chemical bonding, J. Chem. Educ., 85(12), 1680–1685.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Othman J., Treagust D. F. and Chandrasegaran A. (2008), An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Oversby J., (1996), The ionic bond, Educ. Chem., 32(2), 37–38.
- Özmen H., (2004), Some student misconceptions in chemistry: a literature review of chemical bonding, J. Sci. Educ. Technol., 13(2), 147–159.
- Palmquist M. E., Carley K. M. and Dale T. A., (1997), Two applications of automated text analysis: analyzing literary and non-literary texts, in Roberts C. (ed.), Text analysis for the social sciences: methods for drawing statistical inferences from texts and transcripts, Hillsdale, NJ: Lawrence Erlbaum Associates.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301–314.
- Piaget J., (1973), The child's conception of the world (J. Tomlinson, A. Tomlinson Trans.). St. Albans: Granada (Original work published 1929).
- Pilström H., Wahlström E., Lüning B., Viklund G., Aastrup L. and Peterson A., (2007), Modell och verklighet, 2nd edn, Stockholm: Natur och kultur.
- Roth K. J., Druker S. L., Garnier H. E., Lemmens M., Chen C., Kawanaka T., et al., (2006), Teaching science in five countries: results from the TIMSS 1999 video study. Statistical analysis report. Washington, DC: National Center for Education Statistics. Retrieved 14 August, 2012, from http://www.eric.ed.gov/ERICWebPortal/search/detailmini.jsp?_nfpb=true&_&ERICExtSearch_SearchValue_0=ED491193&ERICExtSearch_SearchType_0=no&accno=ED491193.
- Sikorova Z., (2012), The role of textbooks in lower secondary schools in the Czech Republic, IARTEM e-Journal, 4(2), 1–22, Retrieved 15 October, 2012 from, http://biriwa.com/iartem/ejournal/volume4.2/papers/Paper1_Sikorova_IARTEM_RoleTextbook_eJournal_Vol4No2.pdf.
- Taber K. S., (1997), Student understanding of ionic bonding: molecular versus electrostatic framework? Sch. Sci. Rev., 78(285), 85–95.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Taber K. S., (1994), Misunderstanding the ionic bond, Educ. Chem., 31(4), 100–103.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2(2), 123–158.
- Taber K. S., (2003a), Mediating mental models of metals: acknowledging the priority of the learner's prior learning, Sci. Educ., 87(5), 732–758.
- Taber K. S., (2003b), The atom in the chemistry curriculum: fundamental concept, teaching model or epistemological obstacle? Found. Chem., 5(1), 43–84.
- Taber K. S. and Coll R., (2002), Bonding, in Gilbert J., De Jong O., Justi R., Treagust D. and Van Driel J. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer Academic Publishers, pp. 213–234.
- Taber K. S. and Watts M., (1996), The secret life of the chemical bond: students' anthropomorphic and animistic references to bonding, Int. J. Sci. Educ., 18(5), 557–568.
- Taber K. S. and Watts M., (2000), Learners explanation for chemical phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- Taber K. S., Tsaparlis G. and Nakiboğlu C., (2012), Student conceptions of ionic bonding: patterns of thinking across three european contexts, Int. J. Sci. Educ., 34(18), 2843–2873.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2002), Students' understanding of the role of scientific models in learning science, Int. J. Sci. Educ., 24(4), 357–368.
- Tulip D. and Cook A., (1993), Teacher and student usage of science textbooks, Res. Sci. Educ., 23(1), 302–307.
- Yager R. E., (1983), The importance of terminology in teaching K-12 science, J. Res. Sci. Teach., 20(6), 577–588.
| This journal is © The Royal Society of Chemistry 2013 |
