Moving beyond definitions: what student-generated models reveal about their understanding of covalent bonding and ionic bonding
Cynthia J.
Luxford
and
Stacey Lowery
Bretz
*
Miami University, Department of Chemistry and Biochemistry, Oxford, OH 45056, USA. E-mail: bretzsl@miamioh.edu
First published on 2nd April 2013
Abstract
Chemistry students encounter a variety of terms, definitions, and classification schemes that many instructors expect students to memorize and be able to use. This research investigated students' descriptions of ionic and covalent bonding beyond definitions in order to explore students' knowledge about chemical bonding. Using Johnstone's Multiple Levels of Representation framework, an interactive interview protocol was designed to explore secondary and first year university chemistry students' understandings of bonding. This paper reports findings from the first phase of the interview when students were asked to create models to depict and explain covalent and ionic bonding using art supplies. Analysis of the student generated models and their accompanying explanations revealed multiple misconceptions about covalent and ionic bonding even though students could recite definitions.
Introduction
“There's two to three main different types of bonding. Um, ionic and covalent, and then there's metallic. Ionic is between a metal and a nonmetal. Covalent is between two nonmetals.” (Karl, 1st year university chemistry)Karl's descriptions of bonding ring true of the classic distinctions drawn by teachers when introducing bonding. In the textbook Chemistry: The Molecular Nature of Matter and Change(2006), Silberberg presents typical definitions for covalent and ionic bonding:
“Covalent bonding: The idealized bonding type that is based on localized electron-pair sharing between two atoms with little difference in their tendencies to lose or gain electrons (most commonly nonmetals)” p. G-5.
“Ionic bonding: The idealized type of bonding based on the attraction of oppositely charged ions that arise through electron transfer between atoms with large differences in their tendencies to lose or gain electrons (typically metals and nonmetals)” p. G-9.
Several other first year university chemistry textbooks present similar definitions (Brown et al., 2006; Millard, 2008; Tro, 2011). Such definitions are easily memorized by students. How do students understand these definitions? What ideas, if any, do they have about covalent and ionic bonding beyond these memorized definitions? How might chemistry teachers determine how students actually think about covalent and ionic bonding when such memorized definitions can be easily repeated verbatim?
Prior research on conceptions about chemical bonding
Students' misconceptions about chemical bonding have been previously investigated. Chemical bonding misconceptions have been explored through the use of three concept inventories that focused on multiple aspects of bonding (Peterson and Treagust, 1988, 1989; Treagust, 1988; Peterson et al., 1989; Tan and Treagust, 1999; Othman et al., 2008). Peterson and Treagust (1988, 1989) developed a two-tiered concept inventory exploring students' ideas about polarity, shape, intermolecular forces, and the octet rule. Tan and Treagust (1999) developed a second concept inventory by using questions from Peterson and Treagust's inventory as well as from interview data about intermolecular and intramolecular bonds. Othman et al. (2008) developed yet another concept inventory by combining questions from pre-existing concept inventories and prior literature in order to explore students' ideas about bonding and the particulate nature of matter.Several other studies have described students' understandings of covalent bonding or ionic bonding through the use of student interviews (Taber, 1994, 1997, 1998; Schmidt, 1997; Boo, 1998; Coll and Taylor, 2001, 2002; Nicoll, 2001, 2003). These studies found that students' understandings of bonding include misconceptions about bond polarity (Peterson and Treagust, 1989; Coll and Taylor, 2001; Nicoll, 2001, 2003), the shapes of molecules (Peterson and Treagust, 1989), that covalent bond formation involves a transfer of electrons (Taber, 1994, 1997, 1998), that ionic bond formation involves a sharing of electrons (Coll and Taylor, 2001; Nicoll, 2001, 2003), and that the attraction between two oppositely charged ions can be attributed to neutralization (Schmidt, 1997; Boo, 1998). In Taber's work (1997, 1998), students clung to what he termed the ‘octet framework.’ They tended to explain bonding in terms of atoms ‘having,’ ‘needing,’ or ‘wanting’ eight electrons ‘in order to be stable.’
Some studies have used Lewis structures and molecular models to investigate student understanding of bonding. Nicoll (2001) used a series of tasks about the molecule formaldehyde. Students were asked to first define covalent bonding and then to draw a Lewis structure of formaldehyde given the formula COH2. Students were then asked to build a model using Playdoh to show what formaldehyde would look like, which revealed as yet previously unreported misconceptions. Coll and Treagust (2001) found that when undergraduate and postgraduate students were asked to describe or draw the bonding found in common items such as aluminum foil, both groups had a tendency to use simple teaching models learned in secondary school to explain chemical bonding. Cooper and colleagues have documented students' interpretations of Lewis structures (Cooper et al., 2010, 2012) and their extrapolation to properties that they believe are and are not encoded within such structures. Other research studies have shown that physical manipulative models may lead to confusion between ionic bonding and covalent bonding as learners believe a “stick” is an individual covalent bond (Butts and Smith, 1987; Birk and Kurtz, 1999).
There are no reports in the literature, however, regarding students building their own models for the express purpose of comparing and contrasting ionic bonding vs. covalent bonding. Given the importance of predicting properties from models (Cooper et al., 2012), it is important for chemistry instructors to know what students do and do not understand about the models used to depict bonding. This paper describes the methodology of using student-generated models and reports students' explanations of their models as well as analysis of the models and explanations for misconceptions. In particular, the students' models and their explanations are contrasted against their recitation of memorized definitions of covalent and ionic bonding typically provided in textbooks.
Theoretical framework
To explore students' knowledge about bonding through the use of student-generated models, this study was framed using Johnstone's Multiple Levels of Representation, i.e., the macroscopic, submicroscopic and symbolic domains (Johnstone, 1991, 2010), with a focus on the latter two. The macroscopic domain consists of observable properties, e.g., the white, crystalline nature of the solid in a salt shaker or observations made in the laboratory regarding physical or chemical properties. The white crystals would best be described in the submicroscopic domain (also known as the particulate domain) as an ordered lattice of alternating sodium ions and chloride ions and the nature of the interactions between these ions. Lastly, knowledge of this same substance in the symbolic domain would consist of the formula NaCl and attractions between Na+ and Cl− ions known as ionic bonds. In this manuscript, the student-generated models were used to explore the extent to which students made connections between the particulate and symbolic domains. (While the macroscopic domain is important, it was beyond the scope of this particular study.)Research question
In order to surface students' knowledge (and misconceptions) about bonding beyond the recitation of definitions, students were asked to create their own models of covalent bonding and ionic bonding from among a variety of different materials that were provided. This task required students to consider what features were needed in order to depict what they considered to be the essential characteristics of covalent bonds and ionic bonds and distinguish one from the other. The findings reported below answered the question: What do student-generated models of ionic and covalent bonding reveal about students' understandings of ionic and covalent bonding?Student participants
Twenty-four students were interviewed after they had been taught and tested upon covalent and ionic bonding by their teachers. Purposeful sampling (Patton, 2002) ensured students' thoughts about bonding were gathered across a range of student abilities in order to maximize the breadth of ideas while also allowing for a deep exploration of how the students in this study thought about bonding and the models they created. Purposeful sampling prioritizes uncovering the breadth and depth of ideas over the generalizability of findings to a larger population (Patton, 2002). The range of student abilities was maximized by interviewing students enrolled in secondary physical science (SPS), secondary chemistry (SC), and first year university chemistry (UC). All secondary students were minors, and as such, were required to provide both parental consent and verbal assent in compliance with human subjects' approval of this protocol. Eight physical science (SPS) and three secondary chemistry (SC) students were interviewed for the full study. The SPS course consisted of one semester of chemistry followed by one semester of physics; students were interviewed after instruction and during the last week of the first semester and the first few weeks of the second semester. This was the only chemistry course required of students at this rural secondary school; the majority of the students enrolled in this class would go on to take physics or astronomy with only a select few going on to take a full-year chemistry course. Students enrolled in the SC course at a different rural school district were interviewed at the end of their year-long chemistry course. The interviews took place during the last two weeks of the school year while students reviewed for a final comprehensive examination. The thirteen first-year university chemistry (UC) students were interviewed during the second semester of a year-long, non-majors, university chemistry lab sequence. These students had a variety of majors including zoology, biology, and engineering. Students had again all completed instruction and had successfully completed the first semester of the course. This range of courses offered a wide of a variety of student abilities from which to sample.Data collection and analysis methods
Each student was interviewed once (the interviews lasted an average of 60 minutes), using a semi-structured five-phase protocol designed to use multiple representations to generate cognitive dissonance (Linenberger and Bretz, 2012). The protocol was validated using a small pilot study in order to ensure the protocol functioned as intended. The first phase of the interview, which lasted 20 minutes on average, elicited students' prior knowledge about bonding and then asked students to build their own models. Students were provided colored pens, paper, Playdoh and toothpicks to use at any time if they wished to do so. To begin, students were asked to talk about what came to mind when they heard the words ‘chemical bonding.’ When students had finished talking, they were then asked to elaborate upon or clarify any terms they had used. Students were asked questions about representations such as “Who makes representations?” and “What do people have to think about as they make representations?” Students were then asked to build a model of covalent bonding and a model of ionic bonding using the materials provided. Students were encouraged to build multiple models to help clarify their initial model if they felt it would be helpful to do so. The first phase of the interview concluded by asking students to describe how they distinguished between ionic and covalent bonding.The focus of Phase 1 was to elicit students’ ideas without the influence of models typically generated by chemists. The remaining phases of the interview then asked students to interpret such expert representations. The findings reported herein focus on only the first phase of the interview, namely students' prior knowledge and the generation of their own models. Data and analyses stemming from expert generated representations will be published in future manuscripts.
Interviews were both audio and video recorded. The video was used to clarify students' comments during transcription, e.g., about ‘this’ atom or ‘that’ molecule. In order to protect students' identities, pseudonyms were assigned, and students' faces were not captured on video by purposefully directing the camera to capture student gestures (e.g., pointing to particular representations or moving models), but to avoid capturing facial expressions. Interviews were transcribed verbatim using both the audio and the video files. The models built by students were captured on camera. Subsequent screen shots were taken from the video in order to create image files of the models. All data were uploaded into NVivo 8 (QSR International Pty. Ltd., 2007), a data management software program to help with organization and retrieval of the coded interviews. The constant comparative method of data analysis was applied to the data (Strauss and Corbin, 1994, 1998; Taber, 2000). The interviews were first coded using line-by-line open coding, subsequently comparing open codes to one another, and then recoded using a more selective approach focusing on the specific themes that had emerged from the open coding (Lincoln and Guba, 1985; Guba and Lincoln, 1989; Strauss and Corbin, 1998; Patton, 2002; Creswell, 2007). In particular, the interviews were analyzed to examine how students discussed the models they built.
Results
Student ‘definitions’ of covalent and ionic bonding
Students offered a variety of definitions to distinguish between covalent and ionic bonding, but they all were dualistic in nature rather than reflecting a continuum with non-polar covalent bonding at one end and ionic bonding at the other. When asked what they thought of when they heard the words ‘chemical bonding’ at the beginning of the interview, 17 out of the 24 students mentioned the words ‘covalent’ and ‘ionic.’ All but one of the first year university chemistry students immediately talked about covalent and ionic bonding. Only half of the secondary physical science students and one of the secondary chemistry students mentioned the terms covalent and ionic. Across all students interviewed, all but three mentioned the sharing or giving of electrons when describing ‘chemical bonding.’ When asked to elaborate upon what those terms meant, several definitions were typically offered:“Um covalent bonds involve one atom- er two atoms of elements sharing electrons and ionic bonds involve the attraction of charged particles. Um, covalent are typically two non-metals and ionic are typically a metal and nonmetal.” (Kevin, UC)
“Ionic bonding would be I guess where like one atom completely gets rid of an electron making it like… I think it would make it negative. And then it would give it to the other atom one extra electron making it positive… And when they bond covalently where you have two chlorine atoms and they both need one more so they just like… they include each other's one extra er one atom of each other's into the full circle.” (Kyle, SPS)
“Yeah like they can stand alone like ionic bonds I think are usually metals and covalent is like a metal and a nonmetal.” (Billie, SC)
From the data, the classification codes ‘sharing/giving up electrons’ and ‘metals and nonmetals’ emerged based on the commonalities across students as they themselves defined ionic and covalent bonding while building and explaining their models (Table 1). The ‘combined’ classification scheme was created for the five students who mentioned that in order to determine bond type, they would need to know both what atoms were involved and whether the electrons were shared or given away. Additional codes emerged from the data beyond the classification codes and were used to further expand our understanding of how students thought about their bonding models. Explanations invoking electronegativity differences, polarity, and the octet rule were of interest, but only three students discussed electronegativity differences and only two mentioned polarity while building their models. By contrast, 15 of the 24 students discussed the octet rule when describing their models.
| Code | Code definition |
|---|---|
| Sharing/giving up electrons | • Describes/defines covalent and ionic bonding as one involving the sharing of electrons and the other involving transfer (giving up, getting rid of, gaining, etc.) of electrons. |
| • May or may not discuss attraction of atoms rather than transfer. | |
| • Might discuss the type of atoms (metals and nonmetals), however does not use the type of atom to determine or discuss the differences between the two bond types. | |
| Metals and nonmetals | • Describes/defines covalent and ionic bonding based solely on the type of atoms being metals and nonmetals. |
| • May discuss electrons as being either shared or given up, however does not use these features to determine the differences between the two bond types. | |
| • Focus on periodic table trends (metals on left, nonmetals on right). | |
| Combined | • Describes/defines covalent and ionic bonding based on the type of atoms being metals and nonmetals AND one bond type involving a sharing of electrons and the other bond type involving a transfer (giving up, getting rid of, gaining, etc.) of electrons. |
| • Uses both sharing/giving up electrons and metals and nonmetals to classify the bond type as covalent or ionic. | |
| Other | • Does not fit into the three categories above. |
| • Does not mention atoms both sharing and giving up electrons, nor the type of atoms affecting the bond type. | |
Each student was classified based on this coding scheme (Table 2). Inter-rater agreement was established with a second rater who analyzed 6 transcripts randomly selected from the corpus of 24. The classifications were discussed until 100% agreement was reached on these 6 students, and then the remaining 18 participants were coded once more. These categories were used to examine how student-created models and their descriptions thereof aligned (or not) with the definitions of bonding they had offered. Four definitions not fitting these categories, nor supported by textbooks, were grouped and labeled as ‘other definitions’. These four definitions ranged from a student admitting that she was just guessing to one describing each representation with regard to how the atoms in the representation were connected. It is important to note that ideas about bonding beyond the definitions of covalent bonding and ionic bonding emerged as students described their models. Students were categorized in Table 2 based on how they had defined covalent and ionic bonding before they built their models.
Discussion
Turning ‘definitions’ into models
Although a large fraction of the students offered very similar ‘definitions,’ we were interested in what students understood beyond being able to merely recall what they considered to be correct definitions. In order to access this thinking, students were asked to demonstrate their knowledge by building two models – one to show covalent bonding and one to depict ionic bonding. Students were also asked to explain what specific features of their models represented ionic or covalent bonding. Four different categories of models were built across all students (Fig. 1): | ||
| Fig. 1 Examples of models created by students: (a) covalent ‘Ball and stick’ created by April (UC); (b) covalent Lewis structure drawn by Shelly (SPS); (c) ionic electron ‘orbits’ created by Lance (UC); (d) ionic lattice structure created by Nikki (UC). | ||
• ‘ball and stick’ models using Playdoh for atoms and toothpicks as connectors in order to show covalent bonding (Fig. 1a); ‘ball and stick’ model for ionic bonding by either removing the toothpick or by specifying a different meaning for the toothpick.
• Lewis structures for both covalent and ionic bonding (Fig. 1b).
• 3D electron models (orbits) including ‘electron orbits’ or ‘electron rings’ for both covalent and ionic bonding (Fig. 1c).
• Lattice structures in order to explain ionic bonding (Fig. 1d).
While most students built models contained within just one of these categories, seven students built a model in one category for ionic bonding and a different category of model for covalent bonding. Six students built multiple models for one type of bonding, and in doing so, used two or more categories to depict this one type of bonding.
A most interesting finding was that no direct relationship existed between the category of model(s) that a student built and the definitions that s/he offered for covalent and ionic bonding (Table 3). All the models in Fig. 1 were created by students who described and used the ‘sharing/giving up electrons’ classification scheme.
| Sharing/giving up electrons | Metals and nonmetals | Combined | Other | |||||
|---|---|---|---|---|---|---|---|---|
| Covalent | Ionic | Covalent | Ionic | Covalent | Ionic | Covalent | Ionic | |
| Model type | ||||||||
| Ball and stick | 4 | 0 | 5 | 5 | 4 | 2 | 2 | 1 |
| Lewis | 5 | 5 | 2 | 0 | 2 | 0 | 0 | 0 |
| Orbits | 2 | 2 | 1 | 1 | 2 | 3 | 1 | 1 |
| Lattice | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| No model | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Total | 12 | 10 | 8 | 6 | 8 | 5 | 4 | 4 |
| Multiple models | 3 | 1 | 2 | 0 | 2 | 0 | 0 | 0 |
| # Participants | 9 | 6 | 5 | 4 | ||||
Not only did students who offered the same definitions build different models, but as they described their models, some differing misconceptions about bonding also surfaced. Despite being able to ‘recite’ what they thought were standard definitions, students were confused about bonding. For example, one misconception that students held about ionic bonding was that electrons are first transferred and then they become like “a sea of electrons” in ionic compounds (Taber, 2003). Nikki (UC), who provided the sharing/giving up electrons definition for distinguishing between covalent and ionic bonding, built a 3D lattice structure (Fig. 1d) and offered this description:
“Ionic bonding is an array, like, repeating pattern and like the… it's the way we can make a sea of electrons. Electrons kind of like flow through everywhere. They are not being shared like between bonds. The electrons are free to move between each one.” (Nikki, UC).
Although Nikki had defined ionic bonding as “one atom giving up electrons” at the beginning of the interview, when she built a lattice model of NaCl to show ionic bonding, she described ionic bonding as electrons being able to flow from one atom to another. Nikki ignored the ideas of charges and the attractions between ions in her “sea of electrons” explanation. Without the prompts to generate the models and describe how they depicted bonding, this misconception would likely have gone undetected.
Sharing/giving up of electrons
Nine students spoke of ‘sharing/giving up’ electrons while explaining bonding, and they constructed a wide variety of models. A majority of the students offering these definitions (5 out of 9) drew Lewis structures for both covalent and ionic bonding and two students chose to show ‘orbits’. This is the only category for which lattice structures were built as models of ionic bonding. The models all displayed electrons in some way; even the ball and stick models built for covalent bonding included electron(s) being represented by toothpick(s). Of the nine students using this definition, only one student talked about charges and attractions between ions in ionic bonding. He described his lattice structure model of NaCl by saying:“The orange [Playdoh balls] are the Na… the Na plus molecu- er atoms and then the uh blue [Playdoh balls] is the Cl minus in NaCl and um there's like it would be it would be in a crystal form so um the charges of Na the positive charges. That's negative charged so their attracted to each other so they want to be as close as possible.” (Kevin, GC)
The other eight students described ionic bonding in terms of one atom giving or taking an electron from another. While the transfer of electrons is commonly discussed in textbooks to explain ion formation, all but one student described ionic bonding as the transfer or ‘giving up’ of electrons and not as the attraction between charged ions.
Daisy (SPS) provided definitions of sharing/giving and generated two models to explain her understanding. She initially drew a covalent Lewis structure for NaCl (Fig. 2a) and explained, “Chlorine has 7 electrons and sodium has just one, so to form sodium chloride I think that it, yeah, they share them like in the middle…” She then drew an ionic Lewis structure of NaCl (Fig. 2b) and explained, “That would be the taking one… ionic, where like sodium has just one so instead of… to get the perfect 8 instead of sharing they decide to just give it to chlorine.” Daisy thought that NaCl could be either ionic or covalent. When it was pointed out to her that she had drawn NaCl as both ionic and covalent and she was asked which it actually was, Daisy replied, “Either way, I guess they obviously can do it both ways.” Daisy did not understand electron transfer, nor the formation of charges and the attraction between charged ions to form the ionic compound structure.
 | ||
| Fig. 2 Models created by Daisy (SPS) to show (a) covalent bonding and (b) ionic bonding. | ||
These excerpts from Daisy's interview highlight an important finding. While some students may be able to distinguish between sharing electrons and transferring electrons, these same students might not be able to determine whether two atoms will bonded to one another through the sharing of electrons or through ion formation and resulting attractions between ions. Daisy made no mention, for example, electronegativity differences that could be used to decide between the likelihood of covalent vs. ionic bonding. Rather she emphasized the importance of the explicit features of representations such as drawing overlapping circles in Fig. 2a or an arrow in Fig. 2b. To Daisy, the features she drew were what determined the type of bonding between two elements, even to the extent that the same two elements could either form an ionic bonded or a covalent bond to one another. She did not discuss any distinction between metals and nonmetals to help explain her representations. To her, the fact that each model was built using the same two elements was irrelevant. She invoked her prior knowledge of what meanings the arrow and lines conveyed and used these to build her models. Element identity, element symbols and metallic/nonmetallic properties were not critical features for Daisy. Her confusion about covalent and ionic bonding representations revealed that although she could generate some symbolisms used to depict bonding, she showed very little understanding of the ions and atoms that make up compounds.
Metals and non-metals
Although six students provided definitions based on metals and nonmetals to distinguish between covalent and ionic bonding, once again, the models built by these students did not always correlate with their definitions. It is interesting to note that five of the six students built ball and stick models for covalent bonding and for ionic bonding. It is possible that the absence of any electrons in ball and stick models might have been connected to their definitions, however it is also important to note that three of the students did create additional models for covalent bonding that did show electrons, e.g., Lewis structures or orbits.Just as was the case with the students who offered definitions of ‘sharing/giving electrons,’ these students revealed misconceptions while explaining and discussing the specific features of their models, particularly with respect to the electrons in their representations. Diesel (SPS) said that both ionic bonding and covalent bonding involved the sharing of electrons. When asked how he would decide if a bond were ionic or covalent, he said:
“If you know the two types of atoms then two of those would be on the same side, that's ionic. And the one on the… if you have one from the right and one from the left side of the periodic table, that's how you know that the compound is covalent.” (Diesel, SPS)
Although Diesel contrasted the left side and right side of the periodic table, he had the locations of metals and nonmetals reversed. He did have an idea that one type of bonding takes place between a metal and nonmetal and the other between two nonmetals. Diesel then built a model that depicted a covalent bond as one electron being shared “to make eight valence electrons around an outer ring” (Fig. 3a). Curiously, the atom he built with blue playdoh had eight valence electrons and the yellow atom had only 3 electrons. Diesel's model for ionic bonding, however, also showed what he described as electrons being shared in order to make an ionic bond (Fig. 3b). He explained
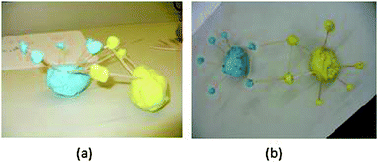 | ||
| Fig. 3 Models created by Diesel (SPS) showing (a) covalent and (b) ionic bonding. | ||
“The electrons are both on the right side of the periodic table so they can't combine… equally so they are sharing from each to get the same amount of electrons.” (Diesel, SPS)
Diesel's models reveal his confusion about what constitutes a bond and how many electrons are involved. In his covalent model, one electron was shared and that one electron itself was the covalent bond. By contrast, the ionic model he generated used two electrons to make a bond, but he described the bond as the attraction being between the two electrons rather than an attraction between two oppositely charged ions. Diesel's ionic model conveys the idea that both atoms have 8 electrons, however he described the bond as being the toothpicks between the two electrons and talked about the electrons being attracted to one another. Despite having previously said that he would need to know what elements are being modeled, Diesel chose not to identify what elements his model was built from despite being asked to do so.
Billie (SC) generated two models for covalent bonding – first a Lewis structure of water and then a ball and stick model of water (Fig. 4). While the ball and stick model was built correctly (Fig. 4b), the Lewis structure lacked the correct number of electrons around oxygen and hydrogen (Fig. 4a) as she drew four electrons around each of the three atoms. Her model also revealed a lack of understanding about the nature of electron pairs, instead drawing them as single, unpaired electrons. Billie's models serve as an important caution for both teachers and researchers that asking students to generate only one type of model may not be sufficient to judge correct understanding and to reveal any misconceptions. If Billie had been asked to generate only the ball and stick model, she might never have struggled with constructing and interpreting the Lewis structure.
 | ||
| Fig. 4 Models created by Billie (SC) showing covalent bonding in H2O through a (a) Lewis structure and (b) ball and stick model. | ||
Combination
Five students commented when building their models that it was necessary to know not only whether a bond was between a metal and nonmetal or between two nonmetals, but also to know whether the electrons were shared or transferred in order to determine whether a bond was ionic or covalent. These five students shared similar ideas and built models similar to those who used only one of these classification schemes. Four students generated ball and stick models for covalent bonding while the fifth student chose to create a Lewis structure. Two students generated multiple models. Two novel misconceptions surfaced as Ryan (UC) and Vanessa (SPS) discussed the movement of particles included in models is an essential characteristic for depicting bonding.Ryan (UC) built a space-filling model of ionic bonding by pushing two spheres of Playdoh together to make one round ball with 2 colored halves. While this model was a bit atypical in that the two ions were each half-spheres, Ryan commented that “together they are one compound, but if they are ions they can float apart.” He explained that what he meant by “float apart” was that if charges were present, the ions could separate from one another on their own without any other interactions. Indeed, he spoke of it as a spontaneous occurrence. He did not picture ion formation as happening until after the ions had “floated apart.” To Ryan, these ions neutralized one another in compounds with one positive ion and one negative ion coming together to form two neutral elements.
Vanessa (SPS) built multiple models to depict covalent bonding, but she chose to not assign identity to the elements. She first built a Lewis structure using generic Os for atoms and smaller circles to show where electrons would be placed (Fig. 5a). She shaded in seven of the electron locations and then further developed the Lewis structure into a 3D electron model using Playdoh (Fig. 5b). She built one atom with an orange nucleus and seven orange electrons. She built a second atom with a yellow nucleus and seven yellow electrons. She demonstrated what she meant by ‘sharing electrons’ by switching one of the orange electrons with one of the yellow electrons and then switching these same two electrons back again. She explained that in covalent bonding, sharing electrons meant that the two electrons “go back and forth” between the two atoms. As with Diesel, she viewed the bond to be an interaction between two specific electrons. She considered the other electrons to be stationary and not involved in the sharing. She did not mention whether these were the only electrons present in the atoms. Although Vanessa could correctly draw a Lewis structure and generate a 3-D model that resembled the Lewis structure, her description of the bonding electrons revealed that she did not understand that all electrons are in constant motion.
 | ||
| Fig. 5 Models created by Vanessa (SPS) for covalent bonding using both a (a) Lewis structure and (b) 3D electron model. | ||
Conclusions
Bonding is traditionally taught emphasizing a dichotomy for molecules and compounds to be classified as either covalent or ionic (Nahum et al., 2010). A more nuanced understanding of bonding beyond the dualistic covalent or ionic is too often “glossed over” in secondary and general chemistry curricula, despite the learning progression that requires a shift from consideration of metals and nonmetals to electronegativity differences and the degrees of polarity (Nahum et al., 2010; Taber, 2011). Research suggests that this dichotomous approach to teaching bonding can be a learning impediment and interfere with further learning about bonding as a continuum (Taber, 1998). None of the students interviewed in this research study made mention of this continuum, choosing instead to offer definitions and build models exemplifying the dichotomy. This bounded thinking no doubt contributed to some of the misconceptions described above.All but one student offered definitions for ionic bonding and for covalent bonding. Although a few students confused covalent bonding for ionic bonding and vice versa, they were able to articulate at least one difference between the two bond types. The most common definitions focused on covalent bonding as the sharing of electrons between two nonmetals and ionic bonding as the transfer of electrons from a metal to a nonmetal.
When students were asked to create their own models of ionic and covalent bonding, several important findings emerged that would not have come to light otherwise. First, there was no direct relationship between the category of models built and the definitions offered for covalent and ionic bonding. Second, misconceptions surfaced despite most students' ability to draw distinctions between metals and nonmetals or between sharing electrons and electron transfer. In addition to confirming several misconceptions already reported in the literature, asking students to generate their own models revealed that some students could not determine whether two atoms would share or transfer electrons when bonded to one another. While only one student discussed ionic bonding as attractions between ions, others described ionic bonds as the attraction between two electrons. Still other students considered the movement of particles included in their models to be an essential characteristic for depicting bonding. These misconceptions were found across multiple model types and definitions offered by the students.
The models also revealed that although students commonly recited a memorized definition for covalent bonding, these students thought of a covalent bond as one atom attracting the electron for a moment, then the other atom attracts the electron, and so on, back and forth between the two atoms. Any understanding of sharing as electrical interactions was missing from the students interviewed, and many of them, like Vanessa (HSPS), clearly showed with their models that they pictured bonding electrons as mobile while nonbonding electrons are not. Ionic bonding was not understood as electrical forces distributed through a lattice by most of the students interviewed. Only three students discussed ionic lattices and only one talked about attraction between ions. Instead, these students clung to the idea that ionic bonding was the actual physical transfer of electrons themselves.
Students created four different types of models: ball and stick, Lewis dot structures, electron orbits, and lattice structures. Overall, the models built across all three groups of students were very similar to each other, and the students held many of the same misconceptions and errors. Seven students spontaneously used more than one model to show one type of bonding in order to further explain additional characteristics of bonding beyond what their initial model showed. Students were encouraged to build additional models, but were not required to do so if they thought that their initial models were accurate.
Implications for teaching
Asking students to create their own models should prove useful in future research studies as it gives the researcher access to students' ideas beyond memorized definitions. Furthermore, asking students to generate more than one type of model may reveal inconsistencies, contradictions, and misconceptions. For this same reason, student-generated models could also equally valuable in the classroom and easily incorporated into instruction through in-class activities. Students' preferences for different types of models may suggest which types of models students are comfortable using (and not). For example, the use of ball and stick models could mask confusion about electrons since the electrons are not typically included in such models. Students could be asked to generate a written description of their models, as well as to clarify what the model can, and cannot, be used to show. Asking students to critique one another's models would provide a focus for discussing the nuances of bonding beyond the parroted, naïve understandings of metals vs. nonmetals and sharing vs. transfer of electrons. Instead, students might be asked to articulate what features are present in their model? What characteristics are essential, but missing from a model built by another student? Does the student's description actually match the model, i.e., can the words in the description be literally matched to various features of the model? The activity of model building with discussion and critique as a community of learners in the classroom will provide teachers at both the secondary and university level a forum to articulate the nuanced understandings of attractions and their importance in bonding.References
- Birk J. P. and Kurtz M. J., (1999), Effect of experience on retention and elimination of misconceptions about molecular structure and bonding, J. Chem. Educ.,76, 124–128.
- Boo H. K., (1998), Students' understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Brown T. L., LeMay H. E. and Bursten B. E., (2006), Chemistry: the central science, 10th edn, Upper Saddle River, NJ: Pearson Prentice Hall.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ.,17, 192–201.
- Coll R. K. and Taylor N., (2001), Alternative conceptions of chemical bonding held by upper secondary and tertiary students, Res. Sci. Tech. Educ.,19(2), 171–191.
- Coll R. K. and Taylor N., (2002), Mental models in chemistry: senior chemistry students' mental models of chemical bonding, Chem. Educ.: Res. Pract. Eur., 3(2), 175–184.
- Coll R. and Treagust D. F., (2001), Learners' mental models of chemical bonding, Res. Sci. Educ., 31, 357–382.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowski M. W., (2010), Lost in Lewis structures: an investigation of student difficulties in developing representational competence, J. Chem. Educ., 87(8), 869–874.
- Cooper M. M., Underwood S. M. and Hilley C. Z., (2012), Development and validation of the implicit information from Lewis structures inventory (ILLSI): do students connect structures with properties? Chem. Educ. Res. Pract.,13, 195–200.
- Creswell J. W., (2007), Qualitative inquiry and research design: choosing among five approaches, 2nd edn, Thousand Oaks, CA: Sage.
- Guba E. G. and Lincoln Y. S., (1989), Fourth generation evaluation, Newbury Park, CA: Sage.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Asst. Lrng.,7, 78–81.
- Johnstone A. H., (2010), You can't get there from here, J. Chem. Educ., 87(1), 22–29.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic inquiry, Newbury Park, CA: Sage.
- Linenberger K. J. and Bretz S. L., (2012), Generating cognitive dissonance in student interviews through multiple representations, Chem. Educ. Res. Pract., 13, 172–178.
- Millard J. T., (2008), Adventures in chemistry, Boston, MA: Houghton Mifflin Co.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Taber K. S., (2010), Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46(2), 179–207.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ.,23(7), 707–730.
- Nicoll G., (2003), A qualitative investigation of undergraduate chemistry students' macroscopic interpretations of the submicroscopic structure of molecules, J. Chem. Educ.,80(2), 205–213.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An investigation into the relationship between students’ conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Patton M. Q., (2002), Qualitative research and evaluation methods, 3rd edn, Thousand Oaks, CA: Sage.
- Peterson R. F. and Treagust D. F., (1988), Students' understanding of covalent bonding and structure concepts, Aus. Sci. Teachers J., 33, 77–81.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students' misconceptions of covalent bonding and structure, J. Chem. Educ.,66(6), 459–460.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade 11 and 12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26, 301–314.
- QSR International Pty. Ltd., (2007), QSR NVivo.
- Schmidt H. J., (1997), Students' misconceptions: looking for a pattern, Sci. Educ.,81, 123–135.
- Silberberg M. S., (2006), Chemistry: the molecular nature of matter and change, 4th edn, New York: McGraw Hill.
- Strauss A. and Corbin J., (1994), Grounded theory methodology: an overview, in Denzin and Lincoln (ed.), Handbook of qualitative research, Thousand Oaks, CA: Sage.
- Strauss A. and Corbin J., (1998), Basics of qualitative research: techniques and procedures for developing grounded theory, 2nd edn, Thousand Oaks, CA: Sage.
- Taber K. S., (1994), Misunderstanding the ionic bond, Educ. Chem., 31(4), 100–103.
- Taber K. S., (1997), Students understanding of ionic bonding: molecular versus electrostatic framework, Sch. Sci. Rev., 78, 85–95.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ.,20(5), 597–608.
- Taber K. S., (2000), Case studies and generalizability: grounded theory and research in science education, Int. J. Sci. Educ.,22(5), 469–487.
- Taber K. S., (2003), Mediating mental models of metals: acknowledging the priority of the learner's prior learning, Sci. Educ., 87(5), 732–758.
- Taber K. S., (2011), Models, molecules and misconceptions: a commentary on “Secondary school students' misconceptions of covalent bonding”, J. Turk. Sci. Educ., 8(1), 3–18.
- Tan K. C. D. and Treagust D. F., (1999), Evaluating students' understanding of chemical bonding, Sch. Sci. Rev., 81, 75–83.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students' misconceptions in science, Int. J. Sci. Educ.,10, 159–169.
- Tro N. J., (2011), Chemistry: a molecular approach, 2nd edn, Upper Saddle River, NJ: Pearson Prentice Hall.
| This journal is © The Royal Society of Chemistry 2013 |
