Chemical reactions: what understanding do students with blindness develop?
Amy L. Micklos Lewis and George M. Bodner*
Department of Chemistry, Purdue University, West Lafayette, IN 47907-2084, USA. E-mail: gmbodner@purdue.edu; Fax: +1 (765) 494-0239; Tel: +1 (765) 494-5313
First published on 19th September 2013
Abstract
This study examined the understanding of chemical equations developed by three students with blindness who were enrolled in the same secondary-school chemistry class. The students were interviewed while interpreting and balancing chemical equations. During the course of these interviews, the students produced diagrams using Braille symbols that provided insight into how they visualized the various components of the equations used to represent chemical reactions. The results of this study suggested that these students all possessed views of the symbolic representations of chemical reactions that differed from those of practicing chemists. The alternative views the students had of these symbols, however, did not differ significantly from the results of previous studies conducted with students with sight. The results of this study suggest that current pedagogical practices should be revised to enhance the conceptual understanding that all students develop of the symbolic representations used to describe chemical reactions.
Introduction
The task of balancing chemical equations plays a crucial role in introductory chemistry courses. In many ways, the concepts developed as this task begins to be understood serve as threshold concepts (Meyer and Land, 2006) upon which fundamental ideas such as the conservation of mass are based. Studies of students' understanding of balanced chemical equations have been shown to provide insight into their understanding of the structure of matter at the atomic or molecular scale (see, for example, Yarroch, 1985; Hinton and Nakhleh, 1999). This study sought to build upon prior work on students' interpretation of chemical equations (see, for example, Yarroch, 1985; Ben-Zvi et al., 1987; Hesse and Anderson, 1992; Garnett et al., 1995; Özmen and Ayas, 2003) by examining the ways students who are visually impaired interpret the symbolic language of chemical equations.Individuals with disabilities in science
Individuals with disabilities are underrepresented in the fields of science and engineering in many countries. Based on U.S. census data, 19.3% of the population has some form of a disability (Waldrop and Stern, 2003). Yet, as of 2006, only 7% of all scientists and engineers had a disability (National Science Foundation [NSF], 2011). This cannot be explained by assuming that students with disability are less likely to pursue higher education. In fact, 70.6% of students with visual impairments and no other disability pursue higher education, whereas only 59.5% of the general population will do so. Although only 3.8% of Americans suffer from blindness or low vision (BLV) (Supalo et al., 2009) instructors of courses in STEM disciplines in the U.S. need to become better prepared to serve these students as the number of BLV students who enroll in their courses increases as a result of legislation that mandates that all students who are cognitively capable of participating in a class should have an opportunity to do so (No Child Left Behind Act, 2002; Individuals with Disabilities Education Act, 2004).Reaching students with blindness and low vision (BLV)
There are few research-based practices available for science educators to use when teaching individuals with visual impairments (Wild and Allen, 2009). Though manuals and articles have been written on how to modify science courses for the visually impaired (Linn and Thier, 1975; Hadary and Hadary, 1978; Ricker and Rogers, 1981; Willoughby and Duffy, 1989; Dion et al., 2000; Koenig and Holbrook, 2000; Kumar et al., 2001) most of these materials are not grounded in research. Some studies addressing changes to the chemistry classroom have been done (Bryan, 1952; Hiemenz and Pfeiffer, 1972; Tallman, 1978; Crosby, 1981; Lunney and Morrision, 1981; Smith, 1981; Tombaugh, 1981; Anderson, 1982; Lunney, 1994; Colwell et al., 2002; Mayo, 2004; Pence et al., 2003; Supalo, 2005; Poon and Ovadia, 2008), but this work often focused on post-secondary education and was based on instructor interactions with a single chemistry student with BLV. Furthermore, this work has not taken an in-depth look at BLV students' interpretations or understandings of specific topics, with the exception of the work done by Mayo (2004).In her dissertation, Mayo (2004) described the results of a study that examined visually impaired university students' visualizations of the structures of common molecular geometries, such as the trigonal planar, tetrahedral, and trigonal bipyramidal structures encountered in discussions of the valence-shell electron-pair repulsion (VSEPR) theory in introductory college-level courses. The subjects of her study were asked to draw simple geometries using a device known as a Thermo-Pen (Repro-Tronics, Inc.) that creates a raised image on what is known as “flexi-paper.” This apparatus allowed these individuals to “feel” the shape of the image they drew. The subjects were given Braille text that described molecular geometries and asked to explain their mental images for each of these structures. Her results suggested that students who were visually impaired had difficulty interpreting common representations of the structures encountered in introductory chemistry and organic chemistry courses.
The role of balancing & representing chemical equations
Studies of students' views of the particulate nature of matter have revealed that students who can successfully balance equations often fail to either identify or create accurate pictorial representations or visualizations (Yarroch, 1985; Nurrenbern and Pickering, 1987; Pickering, 1990; Sawrey, 1990; Nakhleh, 1993; Nakhleh and Mitchell, 1993; Sanger, 2005; Kern et al., 2010). Yarroch (1985) examined above-average secondary school students' experiences with balancing equations and found that a majority of students could not produce accurate pictorial representations because they were unable to interpret subscripts, coefficients, and implicit meanings of the equations. Sanger (2005) came to similar conclusions when using a diagram modified from the work of Nurrenbern and Pickering (1987) to explore undergraduate students' abilities to translate a diagram into a balanced equation and then use it to perform stoichiometric calculations. Only 24 of the 156 participants in Sanger's study were able to identify the correct balanced equation based on a pictorial representation, and 64% failed to demonstrate understanding of the concepts a balanced chemical equation presents. More recently, Kern et al. (2010) found that 65% of the high-school student population could balance an equation, but less than half could produce accurate particulate drawings.Probing students' abilities to move between visual and symbolic modes of representations is important because of the integral role these representational systems play in the sciences, especially in chemistry (Gilbert, 2005). Students with visual impairments cannot utilize many forms of visual and symbolic representations commonly used in the chemistry classroom. Yet, studies have shown that students with vision impairments develop mental images of scientific concepts such as melting, crystallization, combustion, bonding, etc. (Mayo, 2004), and atoms, elements, molecules and matter (Smothers and Goldston, 2009). Our goal in exploring BLV students' abilities to interpret, balance, and present representations of chemical equations was to provide insight into the challenges chemical equations pose for both students who are blind and those who are sighted to enhance access for all students to current representational practices.
Research design
Theoretical framework
This study was based on the theoretical framework of critical theory, which is primarily concerned with issues of uneven distribution of power and justice (Mayo, 2007). Critical theory is transformative; it seeks to produce change in a situation that is likely to remain stable (Brookfield, 2005). It is primarily concerned with issues of power and justice, and assumes that a particular group is being “oppressed” by some function of society (McLaren and Giarelli, 1995; Patton, 2002). Critical theory emphasizes the need to understand the experiences of a group while focusing on creating change for that group within society. This work recognized that the individuals who participated in the study were taught by sighted instructors, using materials created by sighted individuals. It assumed that introductory chemistry courses at both the secondary-school and tertiary levels can serve as a barrier toward careers in many disciplines. It therefore was assumed that the experiences of the students with blindness or low vision needed to be examined if progress was going to be made toward creating pedagogical practices that will provide them with better access to career paths in STEM disciplines.Brookfield (2005) argued that critical theory has five characteristics when applied to educational research. Critical theory is: (1) grounded in a political situation that is unlikely to change, (2) concerned with generating knowledge that can help individuals attain freedom from oppressive aspects of the classroom environment, (3) valid only when participants in the study believe it represents their hopes and dreams, (4) grounded in the existing classroom environment while envisioning a more just world, and (5) a theoretical framework upon which a process is built that continues until a more ideal environment is achieved. No individual research study can achieve these goals. But, as noted by Denzin and Lincoln (2003), critical theory can provide a strategy for exploration of a path toward social justice. This study should therefore be viewed within the context of a long-term effort to facilitate the integration of persons with disabilities into STEM careers.
The two guiding research questions framing this study were:
(1) How do students with blindness or low vision balance chemical equations?
(2) What interpretations do students with blindness or low vision develop about the symbolic representations used to write balanced chemical equations?
Interview design
The results presented in this paper are part of a larger study that used qualitative methods to probe the conceptual knowledge that three students with blindness developed after receiving chemistry instruction in a traditional secondary school. Six interviews were conducted with each student over the 2010–2011 academic year to explore their understandings of the topics of states of matter, atomic structure, nomenclature, the mole, balancing equations, and stoichiometry. The participant interviews averaged 45 minutes in length. The data presented in this paper focuses on interview responses related to the balancing of chemical equations.A semi-structured interview protocol utilizing a think-aloud approach (Ericsson and Simon, 1993; Bowen, 1994) was constructed based, in part, on the chemical equations that had been examined in previous research with sighted chemistry students so that potential comparisons could be made between sighted students and students with blindness. During the interview, the participants were asked to describe how they would balance the following equations:
| N2 + H2 → NH3 | (1) |
| NH3 + H2SO4 → (NH4)2SO4 | (2) |
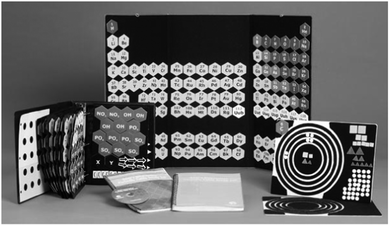
The first equation was presented to the students using Azer's Interactive Periodic Table Study Set (Poppe, 2008) shown in Fig. 1, which contains pieces in different shapes labeled in Braille that can be used to represent chemical equations. The set contains diamond-shaped pieces with the symbol and atomic number for each element, as well as square coefficients, triangular subscripts, and both addition and arrow symbols. To probe the participants' mental models of a chemical equation, they were asked to create a tactile representation of the first reaction using Azer's study set.
The second equation was then presented orally and the participants were asked to balance the equation using the think-aloud technique, and then describe how they would enter the equation into the note-taking technology that they used (e.g., products known as PAC Mate and Braille Lite).
PAC Mate is a pocket PC for blind and low vision (BLV) users manufactured by Freedom Scientific. It provides a version of the Pocket Word software as well as Internet Explorer, Windows Media Player, and the JAWS screen reading software. A Braille display can be added as well as a DAISY player and other applications one would find on a pocket PC. [The DAISY (Digital Accessible Information System) consortium was formed in 1996 to lead the transition from analog to digital talking books.] Freedom Scientific also manufactures the Braille Lite personal digital assistant. It is available as a 20-cell or 40-cell PDA with a built-in modem, adjustable speech output, POP3 Email, word processing capabilities, and software that allows the user to transfer files to a personal computer. Output can be obtained in the form of speech, Braille, or input into a Braille embosser/printer.
Setting
This study took place at a large suburban secondary school with an enrollment of 1900 students located in the Midwestern United States. This school was selected because it was part of a special education consortium that provided access to more than one visually impaired student. All secondary-school students with vision impairments in that county attended this particular school, so that certified vision teachers were available to assist both the classroom teacher and the students with BLV. Although other teachers in the school might have had experience with students with impaired vision, this was the first time students with BLV had been allowed to enroll in a chemistry course at that school. The three student participants were placed in the same classroom by the special education consortium coordinator, so that a certified vision teacher could be available during both a resource period and the chemistry class.The three students were recruited for the study using the Institutional Review Board (IRB) procedures developed for use at Purdue University. Pseudonyms were assigned to the three participants at the beginning of the study. Each individual interview was conducted in a conference room located near their chemistry classroom during the students' study-hall time after they had taken the unit examination on balancing equations.
Participants
David was particularly interested in taking chemistry. During 8th-grade he remembered trying to learn how to balance equations and having his teacher say that the purpose of learning this material was to have “exposure so that when you're in high-school chemistry you won't be out in left field,” and he wanted to put it to the test. David had only been in classes with sighted peers prior to enrolling in this class and had chosen to take an additional year of secondary-school studies to enhance his skills in science, mathematics, and foreign languages. David was consistently an “A” student, but encountered difficulties when balancing equations.
In school, David was most interested in orchestra because he felt that “it's quantifiable.” David was unsure of what he wanted to do after completing high school, but felt that pursuing a career in assistive technology or technical support for the blind would be good because it is important to “give back” to people.
Becky was most interested in her English class because she liked to write. She said that she liked to read as well, but only read the assigned materials for classes. She did not read for her own pleasure. Like David, Becky was unsure of what she wanted to do after high school, but she had picked out a local university to attend because she did not want to be away from home. Becky was vacillating between seeking a career in writing or in music.
Sarah was most interested in English and choir at school. In particular, she felt most successful in choir because she had taken voice lessons and seen improvements in her voice. She was frustrated, however, that she could not sight-read music. Sarah had attempted to learn how to read Braille music, but had not yet conquered its difficulties. After finishing high school, Sarah hoped to pursue a career in music or journalism, where she could combine her interest in music and writing.
Analysis procedure
The first step in the analysis procedure involved transcribing the interviews verbatim. A traditional hermeneutical spiral (Denzin and Lincoln, 1994; Patton, 2002; Samaras, 2011) involving multiple readings of the interview transcripts was used to ground the findings in the interview data. Open coding was then used to determine the key components of the balancing process. Case records were then constructed to highlight key dialogue based on common themes that had emerged. Finally, a constant comparative analysis (Patton, 2002) was performed among the participants' data to draw out similarities and differences.Findings
Reading the symbolic language
The first step in balancing an equation for a student with blindness involves interpreting the tactile symbols so that reactants and products are identified. The following responses were produced when the first equation (N2 + H2 → NH3) was presented to the students using materials from Azer's Periodic Table Study Set. Note that we have chosen not to use a traditional subscripting representation in the transcripts from the interviews because our participants were not able to use this format. Subscripting was only used in the transcription when the participant clearly stated a number was a subscript or clearly placed a number in the correct context for use as a subscript. Because the interviewer worked with the students throughout the year, the interviewer and students developed patterns of communication that allowed her to deduce when subscripts were appropriate in the interview transcript.David read the first equation in a traditional left-to-right fashion, and accounted for the number of atoms of each element that were present on each side of the equation.
“N-2 plus H-2 yields N,H-3 … Alright, so what we've got here. We have two nitrogens on the left. We have two hydrogens on the left. Our lovely little yield sign and then we have, looks like, one nitrogen on the right and three hydrogens on the left, ‘er no, three hydrogens on the right.”
David correctly identified the reactants and product of the reaction. The other participants, however, struggled to discern the information that was necessary when reading from the Azer set. Sarah, for example, also vocalized the atomic number written on the symbol for each element.
Sarah: “It says 7, ah, N, two, ah two. I think you have it upside down.” [Referring to the Braille addition symbol.]
Interviewer: “Do I?”
Sarah: “Possibly.”
Interviewer: “That is a plus sign.”
Sarah: “Oh, plus, never mind.”
Interviewer: “Okay.”
Sarah: “Plus 1, H, and then two and then there is a yields then 7, N, 1, H, 3.”
Whereas David clearly selected the information on each element symbol needed to understand the equation, Sarah was uncertain about which numbers on the element symbol were useful, and struggled to read the equation.
After the interviewer noted that the “7” and “1” referred to the atomic numbers for nitrogen and hydrogen that were written on the symbols for the elements in the Azer study set, Sarah was asked to count the numbers of each atom present but was uncertain about the difference between a subscript and a coefficient.
Interviewer: “Okay, so how many atoms of nitrogen are there?”
Sarah: “Two”
Interviewer: “Can you tell me, is that a coefficient, subscript, or superscript?”
Sarah: “I'm guessing, now that you say that, probably a coefficient. Oh, wait, it is not in parentheses, those are…”
Sarah: “What's the difference?”
Interviewer: “Between a subscript and a coefficient?”
Sarah: “Yeah.”
Although David seemed to read the symbols for the elements correctly, he also asked for clarification about subscripts, noting that he was not confident in his understanding of the difference between subscripts and superscripts.
David: “Alight, you've got a subscript; I believe it is a subscript. Is that accurate? You really can't tell between superscripts and subscripts in Braille, I know we are supposed to know, but I never knew the difference really.”
The inherent limitations of Braille, in one case, and the Azer study set, in the other, created an additional hurdle in understanding the implications of the terms subscript, superscript, and coefficient.
The discussion of the addition sign and the arrow used in both the first and second equation suggested that the participants easily identified and understood the way these symbols were being used. During this part of the interview it also became apparent that the students felt no need to refer to nomenclature previously learned. For example, Becky read the reaction as follows:
Becky: “Element plus element and then the arrow is yields or reacts with, I mean produces. The plus is reacts with.”
Interviewer: “The plus is reacts with and the arrow means produces?”
Becky: “Yeah.”
Interviewer: “Do you know all the names of all the chemicals present?”
Becky: “Well, I know N is nitrogen.”
Interviewer: “Okay.”
Becky: “And then the H is hydrogen and then nitrogen again. Yeah, so yeah, it is nitrogen and hydrogen.”
Interviewer: “Is NH3 a particular chemical?”
Becky: “Not that I know of.”
Becky was able to describe in basic terms how the arrow and addition signs were relevant to the chemical equation, but she did not attempt to name any of the elements or compounds present.
Sarah was able to provide more detail in her discussion of the addition and arrow symbols during her interview.
Interviewer: “… Can you explain to me what the plus sign means, when you said plus, what does that mean?”
Sarah: “It means that they're put together. That's how I look at it. Not exactly the same molecule, but their combined in some way.”
Interviewer: “And then you said, yields, and what symbol represents the yield?”
Sarah: “The arrow.”
Interviewer: “And what does yield mean?”
Sarah: “Makes.”
Interviewer: “Okay, anything more about makes?”
Sarah: “Reacts with, like it's the product. This is the reactant [pointing toward the left side of the equation] and then here is the product side” [pointing toward the right side of the equation].
Interviewer: “And this reaction produces what?”
Sarah: “It produces 7 N and 1 H-3.”
Sarah understood the difference between the reactants and the products of the reaction. To her, the plus sign indicated that the molecules of N2 and H2 were combined in some way, and the arrow indicated that something was produced when this happened. Like Becky, Sarah did not refer to NH3 as ammonia, but referred it in terms of information she read from the Azer study set symbols.
David was more explicit in explaining the use of the addition and arrow symbols and emphasized the role they played in his atom counting scheme, which suggested that he understand how these symbols were used to represent the reactant and product sides of the equation. Yet, he too appeared to have difficulty articulating the nomenclature that would be used to describe the components of the reaction.
Interviewer: “What does the plus sign mean?”
David: “It is reacting with; it is combining with something, to make something else. It is combining with hydrogen and I look down and I see there are two of these hydrogens. That looks great. Okay. So, this yields sign, you combine nitrogen and hydrogen you make, yields, ah, one N, or NH-3. And I can tell it is a three because there is a little number three under the hydrogen.”
David clearly stated that the plus sign indicated that things were “reacting” or “combining,” and that the arrow was used to indicate that NH3 was produced in the reaction, but he did not identify any component of the reaction by name. Like Becky and Sarah, David referred to the reactants and products solely in terms of the number of atoms present in each component of the reaction.
The process of “reading” a tactile chemical equation is significantly different from reading a traditional script equation because of the additional step required to identify subscripts and coefficients from the Braille representation of the equation. David and Sarah explicitly noted that it was difficult to distinguish between a subscript and a coefficient in Braille. This is not a problem that is unique to visually impaired students; Yarroch (1985) noted that the sighted students in his study confused the meaning of the subscripts and coefficients in a balanced chemical equation. But the task of differentiating between subscripts and coefficients is made more difficult by the technology used to represent a chemical equation in Braille.
The balancing process
The students continued to express difficulty discerning how to use coefficients and subscripts while discussing the process of balancing the second equation [NH3 + H2SO4 → (NH4)2SO4]. When David described balancing the second equation, he occasionally changed the way he kept track of the number of atoms of each element in the equation.David: “Okay. Um, we've got to get a count of what we've got here. We have, one nitrogen on the left and we have four hydrogens, or no, five hydrogens, right, because we have an H3 and an H2. We've got five hydrogens. One sulfur, four oxygens. On the right we have two nitrogens, eight hydrogens, one sulfur and four oxygens.”
Interviewer: “Okay.”
David: “So, NH3, that needs, first of all that, we need to make that a two NH3 because we need two nitrogens. I want to get the nitrogens worked out first. We have two nitrogens on the right and one on the left. So, we come up with two. We put a two in front of that. In front of the capital N, so two NH3, now I have, that is essentially now is two NH3 and ah, let's see I've got, at least my SO4 is fine. I don't have to mess with that, at least not yet. Ah, now I have eight hydrogens on the left, I think, no hang on. Two and then H3, is H6, because the two is connected to the nitrogen that I had to multiply by two is connected to the hydrogen, the H3, right?”
David started by identifying an H3 and H2 on the left side of the equation, for a total of five hydrogen atoms. When he accounted for the coefficient of two that had to be placed in front of the NH3 on the left side of the equation, he referred to the hydrogen atoms as H6 instead of six hydrogen atoms. When he did this, David exhibited uncertainty about the implications of the subscript and the coefficient. This may explain some of the difficulty he had when creating a pictorial representation of the reaction, which will be discussed later.
Sarah exhibited more trouble understanding the mathematical implications of the subscript and coefficient.
Sarah: “… My only question is when I, so if I have five [hydrogen atoms] on the left side and eight on the right side, could I add a three, like five plus three to make it eight?”
Interviewer: “You cannot add five plus three to make it eight.”
Sarah: “Oh.”
Sarah: “I meant, because of the H-3 and the H-2 make five, and then the four in the parentheses and the two outside of the parentheses makes it eight.”
Interviewer: “Okay, was that adding or multiplying?”
Sarah: “Oh.”
Interviewer: “The only thing you can do here is multiply, and what did we call that number?”
Sarah: “The coefficient.”
Interviewer: “And where was the coefficient?”
Sarah: “Oh, it's the four?”
Interviewer: “It's before?”
Sarah: “No, I mean, I'm sorry it is the number four, NH-4.”
Sarah missed a pivotal piece of information about what she could change when she was balancing a chemical reaction. It seemed that she did not understand what she could change to balance the equation because her understanding of the nature of subscripts and coefficients was weak. The interviewer tried to provide step-by-step recommendations on how to determine how many hydrogen atoms were present on the reactant side of the equation, but Sarah still could not apply it.
Sarah: “So, now I have two NH3 plus H2 capital S, capital O4, yields parentheses NH4 close parentheses, capital S, capital O4.”
Interviewer: “Okay.”
Sarah: “My only question is how to get that eight [hydrogen atoms on the reactant side of the equation].”
Interviewer: “Okay. Well, let's talk about how many hydrogens you have on the left side now?”
Sarah: “Five” [quick and confident]
Sarah initially added the subscripts present on the hydrogen atoms. When the interviewer asked her to account for the coefficient's effect on the ammonia species and then added the hydrogen atoms from the sulfuric acid she tried to continue as if she was only dealing with coefficients.
Interviewer: “But, you said you put a coefficient two in front of NH3?”
Sarah: “Because there were two nitrogens on the right side, it was parentheses NH4 close parentheses two. You multiply everything.”
Interviewer: “So, now nitrogen is balanced. So now we move on to hydrogen and you said there are two NH3, so how many hydrogens are in that 2NH3?”
Sarah: “There is H3 plus H2.”
Interviewer: “We don't worry about the H2 yet. We are just worrying about the NH3 right now.”
Sarah: “Oh, technically six.”
Interviewer: “So, now we have six H's in NH3 and now we look at the plus sign.”
Sarah: “H2.”
Interviewer: “Okay, so now you add the H2 to it. So how many hydrogens are there on the left side all together now?”
Sarah: “Twelve?”
Interviewer: “Explain?”
Sarah: “Because we multiply six times two.”
Sarah was lost in a subscript/coefficient tangle in which she had difficulty determining a pattern for the mathematics that applied to the subscripts and coefficients.
Becky's attempt at balancing the second equation were similar to Sarah's because she questioned what she could to do to account for differences in the number of atoms of an element on different sides of the equation.
Interviewer: “Go ahead and talk me through how to balance the equation.”
Becky: “We'll go through each element. So, like there is one, wait, I think there is one N on the left and then see then I get confused with the one's in parentheses, ‘cause like the subscript two, is that referring to the whole thing of NH4?”
Interviewer: “Yes it is. Good. So, how many nitrogens do you have on that side?”
Becky: “Isn't it eight because of the two and the four?”
Interviewer: “Okay. So, what's the number on the N originally in the parentheses?”
Becky: “NH4.”
Interviewer: “Okay, so if there is a two on the outside [of the parentheses] and there is only one N on the inside [of the parentheses] how many?”
Becky: “Two.”
Interviewer: “So, we have two over on that side” [referring to the product side].
Becky: “Wait; see that's when I get confused about what numbers to add, like when you balance them, ‘cause I got like two different things.”
Becky first questioned the role the subscript has when applied to symbols inside parentheses: “… is that [the subscript two applied to contents of the parentheses] referring to the whole thing of NH4?” She then struggled with the question of whether the subscript “4” inside the parentheses in (NH4)2SO4 applied to the nitrogen atom because she stated that there were eight nitrogen atoms because of the “2” outside of the parentheses and the “4” inside the parentheses. Throughout the remainder of the interview, it was clear that Becky did not understand the implications of the subscripts outside the parentheses.
It was clear throughout Becky's and Sarah's interviews on balancing chemical equations that they struggled to decipher what mathematical process to utilize based on the location of the subscript and the implications of the coefficient. Becky and Sarah often questioned their own thought processes and frequently asked the interviewer for guidance. In the end, all three students successfully balanced the equation, but this only happened when Sarah and Becky were guided by the interviewer's questions.
Representations
Each of the students was asked to create a representation of the first equation based on the balanced equation he or she produced. When asked to do this, David responded that he did not think of chemical formulas in terms of connectivity of the components, but he agreed to create a diagram based on his ideas. Both David and Sarah asked for clarification on what the interviewer meant when she asked them to use pieces from the Azer's study set to show them “what the components of the equation look like.” David's pictorial representation of the first equation is shown in Fig. 2. | ||
| Fig. 2 David's representation of the reaction described by the first equation. | ||
David's representation of the reaction symbolized by the first equation suggests that he understood that certain molecules are diatomic.
Interviewer: “Do you think they are connected, how are the N2's ‘hanging out?’”
David: “They should be connected. Like this, because there are two of them and there is nothing in between them.”
David represented the N2 molecules on the left side of the equation as if they were touching, but he felt there was no reason for connecting them other than they were all N2 molecules. He then explained the representation he created for the products in terms of a distributed property he carried over from mathematics, and depicted the products of the reaction in terms of two N2 molecules and two H3 molecules.
David: “Right now I have two nitrogens on the left and two nitrogens on the right. We're happy, okay, but I have two hydrogens on the ri-, that makes, that makes, this nitrogen and hydrogen on the right side, they are combine. It's one thing, so if I have two nitrogens, if I have two, that multiplies over to the hydrogen as well as the nitrogen. So, I have two H-3's. That's like saying I have six hydrogens total. Okay.”
David argued that the coefficient two “multiplies over” the nitrogen and hydrogen atoms of ammonia, which is how he portrayed it in his representation.
David balanced the equation and demonstrated an understanding of the mathematical implications of the coefficient and subscript he used. However, his representation of the balanced equation suggests that he was struggling to determine the interactions and connectivity on the atomic/molecular scale that are implied in the chemical equation. Even though he stated that the nitrogen and hydrogen atoms of NH3 “are combine,” he did not represent these atoms as being attached in any way.
Sarah was the only participant to use the terms compound, and molecule in an unprompted fashion. Moreover, she was the only participant to attempt to create logical connections between her representation of the first equation, shown in Fig. 3, and content from class that extended beyond accounting for the number of atoms present.
 | ||
| Fig. 3 Sarah's representation of the first equation. | ||
Interviewer: “Now, what I want to do is have you show me about what you had there. Okay.”
Sarah: “What do you mean?”
Interviewer: “When this [the balanced equation] says N-2, I'm going to hand you two nitrogens and above the problem is some blank space, how do you think those go together?”
Sarah: “Um,” [placing N–N].
Interviewer: “Okay, are they touching for any particular reason?”
Sarah: “Well, I was thinking of HONClBrIF.”
Interviewer: “Good, so what is HONClBrIF?”
Sarah: “HONClBrIF is the acronym for the molecules that go in pairs.”
Interviewer: “Okay.”
Sarah: “I want to symbolize that they go together, which is why I have them touching.”
Interviewer: Since they are touching each other what word would you use to describe that from chemistry class?”
Sarah: “Compound.”
Interviewer: “It's a compound, okay, is there anything else?”
Sarah: “They're a gas.”
Interviewer: “Are they bonded together?”
Sarah: “Yes! A chemical bond.”
Interviewer: “They have a chemical bond between them.”
Sarah understood that some substances were diatomic molecules. She used the acronym HONClBrIF to identify substances that were likely to form diatomic molecules. She initially identified these substances as molecules, but when asked to verify her word choice she called them compounds, which suggests some confusion about the difference between a compound and a molecule.
Much like David, Sarah represented the product in an unconventional method. She started by connected the two nitrogen atoms. She then added six hydrogen atoms, some of which were connected to the nitrogen atoms. The result was a representation that reflects confusion about the implications of both the subscripts and coefficients in the balanced equation, as shown in the following dialogue.
Interviewer: “Okay, in your balanced equation what is the two, what does that tell us about it when we have two NH3, what does that mean?”
Sarah: “Two N's and then three H's.”
This dialogue reflects her confusion regarding the implications of the coefficient. She applied the coefficient to just the nitrogen atom of the NH3 formed in the reaction. Upon additional questioning, she recognized that the coefficient affects the number of hydrogen atoms.
Interviewer: “Does that [the coefficient on ammonia] change how many hydrogen atoms there are?”
Sarah: “Yeah.”
Interviewer: “How many hydrogens are there?”
Sarah: “Well, there are three right now, but there is supposed to be six.”
Sarah caught her own mistake, but only with some guidance from the interviewer.
It is clear from Sarah's representation of the first equation that she thought the coefficient created one large molecule or compound, instead of two NH3 molecules. Sarah also noted that she modified her diagram to contain representations of diatomic molecules on both sides of the equation, not realizing that the product of the reaction no longer required such action.
Interviewer: “And are these attached [referring to the hydrogen atoms of the large 2NH3 structure]?”
Sarah: “They are supposed to be.”
Interviewer: “That helps out. Could the hydrogens be attached to the nitrogens or just other hydrogens?”
Sarah: “I think they should be attached to other hydrogens.”
Interviewer: “Any particular reason?”
Sarah: “Because they all go together.”
Interviewer: “How come?”
Sarah: “HONClBrIF.”
Interviewer: “Okay, alright.”
Sarah's explanation of her diagram was more detailed than David's, but it still indicated confusion about the connectivity of atoms in a compound.
Although Becky could not produce a balanced equation for the first reaction, she was asked to create a diagram and discuss the components of the unbalanced equation. As shown in Fig. 4, Becky's representation does not connect any of the atoms.
 | ||
| Fig. 4 Becky's representation of the first equation. | ||
Becky explained that no atoms were connected, but atoms that were similar should be closer together than atoms that were different.
Interviewer: “Are they [referring to N2 of the reactant] going to touch each other or are they far apart?”
Becky: “They're going to be close together” [referring to N2 of the reactant].
Interviewer: “They are close together?”
Becky: “I would assume so.”
Interviewer: “Are they touching or connected?”
Becky: “Maybe we never really went over that. They're probably like this, close together.” [Pointing toward the obvious space between the two nitrogen atoms]
Interviewer: “Do they touch?”
Becky: “No.”
Interviewer: “Why not?”
Becky: “Because it is two separate ones not one connected thing.”
Interviewer: “Okay. How about H2, how do you think that would look?”
Becky: “The same.”
Interviewer: “I'll give you two H's.”
Becky: “Maybe like this.” [Placing the two hydrogen atoms close together, but not touching]
Interviewer: “Alright. Let's do one more, now how about NH3? I'll give you the N and thee hydrogens and show me what it looks like.”
Becky: “I feel like they would be all close together, maybe like the nitrogen would be more separated and then since it is H3, there is like three hydrogens.” [Placing the nitrogen atom far from the three hydrogen atoms]
For Becky, the chemical formula does not express any information about the relationship between the atoms or their bonds. But Becky used the subscripts to identify how many of each atom should be present.
All three subjects of this study struggled to create representations that expressed a full understanding of the components of a chemical equation. David and Sarah were able to demonstrate that diatomic molecules were present. Sarah was the only individual, however, to express a logical reason for presenting some of the molecules as diatomic. But Sarah and David were not able to recognize that the product did not require linking atoms in a diatomic fashion. Sarah was able to articulate her ideas using more of the language of chemistry than the other participants. Upon questioning, however, Sarah had difficulty verifying what the terms compound and molecule mean. The notable feature of all the representations was an incomplete or inaccurate visualization of bonding on the atomic/molecular scale.
Writing equations in Braille
The data collected in this study clearly suggests that it is difficult to write a balanced equation in Braille because it is difficult to differentiate between subscripts, superscripts, and coefficients. Although there is a well-developed mathematical language in Braille known as Nemeth Code (Nemeth, 1972) for encoding scientific and mathematical notation using the standard six-dot Braille convention for tactile processing by the visually impaired, students may have difficulty applying Nemeth Code to chemistry, either because they are unaware of how to use it or because they are discouraged from applying it. Each of the participants in this study explicitly noted the difficulty they encountered in accessing the symbols they encounter in science. David, for example, described how he wrote out the second equation in his note-taking tool (PAC Mate).David: “Okay, what I have is capital N, capital H, number thee, plus, capital H two, capital S, capital O, four and then to make the yield sign I do dash, dash, and then the greater than sign. That is what [the certified vision teacher] recommended. Open parentheses, capital N, capital H, H four, close parentheses two, capital S, capital O, four.”
David did not indicate the use of a Braille code for a subscript. Becky noted that neither she nor David were encouraged to use a Braille code for subscripts.
Becky: “Well, I don't know why, but [the certified vision teacher] says we don't have to write the subscript signs.”
Interviewer: “Okay, is there a way for you to write the subscript in there?”
Becky: “No, but I think there is one for superscript.”
Interviewer: “You can write a superscript, but not a subscript?”
Becky: “Yep, I don't know why.”
Sarah used a note-taking technology (Braille Lite) that has only a Braille keyboard and she could not print off her work for the chemistry teacher to review. Her work had to be interpreted and then transcribed by a vision teacher.
Sarah: “Yeah, the only problem is that [the certified vision teacher] has to transcribe it and he doesn't like it very much.”
The additional step required before Sarah's work could receive feedback created a delay in the chemistry teacher's ability to address alternative conceptions during formative assessments. Sarah's technology created another problem as well.
Sarah: “Well, one thing I tend to do with the Braille Lite, just because it is harder for me to insert and delete things and put them back in again, is I try to balance it in my head because it is just easier that way.”
Sarah's struggle to insert and delete numbers meant she had no “scratch paper” to keep track of her changes to an equation and therefore she tried to mentally different combinations of numbers. However, the Braille Lite did allow Sarah to use a notation for subscripts.
Discussion
The results of this study of the process students with BLV use to balance chemical equations suggests that these students balance equations in much the same way as their sighted peers; as nothing more than an algebraic exercise. The results were also consistent with the work by Yarroch (1985), who found that sighted students struggle with the chemical implications of a balanced equation for a reaction. While it appears that both students with BLV and sighted students develop an understanding of the chemical symbols, the plus sign, and the arrow in the equations used to represent chemical reactions, they do not associate this representation with a clear or well-articulated image of the connectivity between atoms in a compound (Yarroch, 1985; Smith and Metz, 1996; Sanger, 2005; Kern et al., 2010). Although the number of participants in this study was small, our data suggests that students with blindness might be more likely than sighted to have difficulty comprehending the implications of subscripts and coefficients and the symbolic meaning of chemical formulas in a balanced chemical equation due to the limitations of the technology they bring to the chemistry classroom.Yarroch (1985) recommended restructuring instruction to emphasize the importance of subscripts in chemical formulas and coefficients in balanced equations. Similarly, Kozma and Russell (1997) called for explicit instruction on the underlying assumptions present in representations of chemical phenomenon. Since students with blindness are encountering similar difficulties as their sighted peers, it might be useful for all students to manipulate tactile models as part of the content instruction that focuses on chemical formulas and balanced chemical equations. These tactile models might provide useful, explicit connections between the symbols in a chemical equation and changes in the connectivity of atoms that occur in the reaction the equation represents. They might also enhance students' comprehensions of the implied chemical bonds that chemical formulas represent. Using tactile models may lead students to find meaning in chemical equations that go beyond lists of letters and numbers (Bodner and Domin, 2000; Shane and Bodner, 2006). For students to establish connections between the components of a balanced equation, they need explicit experiences that facilitate the development of logical patterns for manipulating coefficients and a stronger understanding of subscripts, and bonding. Such experiences may offer students opportunities to create more accurate mental representations and lead to a greater understanding of what happens in a chemical reaction on the atomic/molecular scale.
The results of this study suggest that science teachers and special education teachers need to consider the symbols scientists use within the context of recognizing that they might not be easily represented in the same fashion for the increasing number of students with blindness and low vision who choose to take their courses. Representations of many processes analogous to chemical equations may require additional contemplation so that both students with blindness and sighted students develop legitimate mental models of these processes.
An unanticipated result of this study was brought to our attention by the special education consortium that provided access to the study participants. They noted that tools such as Azer's Periodic Table Study Set have become readily available because government agencies will often subsidize their purchase. When a tool of this nature is used, students with blindness need time to familiarize themselves with it. In this study, the tool was used very sparingly with the study participants because it was bulky and often frustrating because it was a multi-purpose tool that contained several different kinds of information. Simple tools for teaching chemistry to students with blindness have been developed (Graybill et al., 2008) and will be described in more detail elsewhere.
Conclusion
Studies of both sighted students and our sample of students with blindness suggest that chemistry instructors need to consider modifying their pedagogical practices for helping students learn the concepts involved in the task of balancing chemical equations. Part of the problem is that students do not “see” the meaning behind the symbolism contained within these equations that chemists and other scientists take for granted. In particular, students miss the meanings behind the subscripts, coefficients, and the connectivity of atoms represented by the symbols in chemical formulas. In the language we used more than 25 years ago (Bodner, 1986), “Teaching and learning are not synonymous; we can teach, and teach well, without having the students learn.” As chemists, we know what we want students to learn. Yet we may not be scaffolding this learning by providing representations students with or without sight can use. For the vast majority of our students who will not pursue chemistry as a career, we need to help them construct mental models of the world in which they live—on both the macroscopic and molecular scales—they can use to understand and communicate to others fundamental patterns in the world in which we live.We contend that studies, such as this, of the problems that students with disabilities encounter may provide insight into new ways of approaching the task of teaching and learning chemistry that would be useful for students without disabilities, as well. The results of this study, in particular, suggest that we need to provide students with experiences that are more tangible. It might also suggest that valuable insight into the problems of teaching and learning might be obtained by asking pre-service teachers to reflect on how they would present information to students who are blind.
References
- Anderson J. L., (1982), Chemical instrumentation for the visually handicapped, J. Chem. Educ., 59(10), 871–872.
- Azer's Periodic Table Study Set (2008), American Printing House for the Blind, Inc., last accessed 15 September 2013, http://shop.aph.org/webapp/wcs/stores/servlet/Product_Azer's%20Interactive%20Periodic%20Table%20Study%20Set_1-08856-00P_10001_11051.
- Ben-Zvi R., Eylon B. and Silberstein J., (1987), Students' visualization of a chemical reaction, Educ. Chem., 24, 117–120.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 83, 873–878.
- Bodner G. M. and Domin D. S., (2000), Mental models: the role of representations in problem solving in chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Bowen C. W., (1994), Think-aloud methods in chemistry education: understanding student thinking, J. Chem. Educ., 71(3), 184–190.
- Brookfield S. D., (2005), The power of critical theory: liberating adult learning and teaching, San Francisco: Wiley Imprint.
- Bryan A. H., (1952), Chemistry for the blind, Sci. Educ., 36(2), 91–95.
- Colwell C., Scanlon E. and Cooper M., (2002), Using remote laboratories to extend access to science and engineering, Comput. Educ., 38(1), 65–76.
- Crosby G. A., (1981), Chemistry and the visually handicapped, J. Sci. Teach., 58(3), 206–208.
- Denzin N. K. and Lincoln Y. S., (1994), Introduction: Entering the field of qualitative research, in Denzin N. K. and Lincoln Y. S. (eds.), Handbook of qualitative research, pp. 1–17.
- Denzin N. K. and Lincoln Y. S., (2003), Strategies of qualitative inquiry, 2nd edn, Thousand Oaks: Sage Publications, Inc.
- Dion M., Hoffman K. and Matter A., (2000), Teacher's manual for adapting science experiments for blind and visually impaired students, Retrieved April 13, 2010, from http:www.tsbvi.edu/Education/Manuae2.doc.
- Ericsson K. A. and Simon H. A., (1993), Protocol analysis: verbal reports as data, revised edn, Cambridge: MIT Press.
- Garnett P. K., Garnett P. J. and Hackling M. W., (1995), Students' Alternative Conceptions in Chemistry: A Review of Research and Implications for Teaching and Learning, Stud. Sci. Educ., 25(1), 69–96.
- Gilbert J. K., (2005), Visualization: a metacognitive skill in science and science education, in Gilbert J. K., (ed.), Visualization in Science Education, Dordrecht: Springer, vol. 1, pp. 9–29.
- Graybill C. M., Supalo C. A., Mallouk T. E., Amorosi C. and Rankel L., (2008), Low-cost laboratory adaptations for precollege students who are blind or visually impaired, J. Chem. Educ., 85(2), 243–247.
- Hadary D. F. and Hadary S. H., (1978), Laboratory science and art for blind, deaf, and emotionally disturbed children, Baltimore: University Park Press.
- Hesse J. J. and Anderson C. W., (1992), Students' conception of chemical change, J. Res. Sci. Teach., 29(3), 277–299.
- Hiemenz P. C. and Pfeiffer E., (1972), A general chemistry experiment for the blind, J. Chem. Educ., 49(4), 263–265.
- Hinton M. E. and Nakhleh M. B., (1999), Students' microscopic, macroscopic, and symbolic representations of chemical reactions, Chem. Educ., 4(5), 158–167.
- Individuals with Disabilities Education Improvement Act of 2004, H.R. 1350 (2003).
- Kern A. L., Wood N. B., Roehrig G. H. and Nyachwaya J., (2010), A qualitative report of the ways high school chemistry students attempt to represent a chemical reaction at the atomic/molecular level, Chem. Educ. Res. Pract., 11, 165–172.
- Koenig A. and Holbrook M., (ed.), (2000), Foundations of education volume II: instructional strategies for teaching children and youths with visual impairments, 2nd edn, New York: AFB Press.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Kumar D., Ramassamy R. and Stefanich G., (2001), Science for students with visual impairments: teaching Suggestions and policy implication for secondary learners, Electronic Journal of Science Education, 5(3), 1–9. Retrieved from http://ejse.southwestern.edu/article/viewArticle/7658/5425.
- Linn M. C. and Thier H. D., (1975), Adapting science material for the blind (ASMB): expectation for student outcomes, Sci. Educ., 59(2), 237–246.
- Lunney D., (1994), Development of a data acquisition and data analysis system for visually impaired chemistry students, J. Chem. Educ., 71(4), 308.
- Lunney D. and Morrision R. C., (1981), High technology laboratory aids for visually handicapped chemistry students, J. Chem. Educ., 58(3), 228–231.
- Mayo P., (2004), Assessment of the impact chemistry text and figures have on visually impaired students' learning, Doctoral Dissertation, Purdue University, West Lafayette.
- Mayo P., (2007), Critical theory, in Bodner G. M. and Orgill M., (ed.), Theoretical frameworks for research in chemistry/science education, Upper Saddle River, NJ: Pearson Education, pp. 243–261.
- McLaren P. and Giarelli J., (1995), Critical theory and educational research, New York: State University of New York Press.
- Meyer J. H. F. and Land R., (2006), Threshold concepts and troublesome knowledge (2): epistemological considerations and a conceptual framework for teaching and learning, Higher Educ., 49, 373–388.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual students in general chemistry, Chem. Educ., 70(1), 52–55.
- Nakhleh M. B. and Mitchell R. C., (1993), Concept learning versus problem solving: there is a difference, J. Chem. Educ., 70(3), 190–192.
- National Science Foundation [NSF], (2011), Women, minorities, and persons with disabilities in science and engineering, Retrieved April 10, 2011, from http://www.nsf.gov/statistics/wmpd/figh-8htm.
- Nemeth A., (1972), The Nemeth code for mathematics and scientific notation, Frankfort American Printing House for the Blind.
- No Child Left Behind Act of 2001, Pub. L. No. 107–110, § 115, Stat. 1425 (2002).
- Nurrenbern S. C. and Pickering M., (1987), Concept learning versus problem solving: is there a difference, J. Chem. Educ., 64(6), 508–510.
- Özmen H. and Ayas A., (2003), Students' difficulties in understanding of the conservation of matter in open and closed-system chemical reactions, Chem. Educ. Res. Pract., 4(3), 279–290.
- Patton M. Q., (2002), Qualitative research and evaluation methods, 3rd edn, Thousand Oaks: Sage Publications, Inc.
- Pence L. E., Workman H. J. and Riecke P., (2003), Effective laboratory experiences for students with disabilities: the role of a student laboratory assistant, J. Chem. Educ., 80(3), 295–298.
- Pickering M., (1990), Further studies on concept learning versus problem solving, J. Chem. Educ., 67(3), 254–255.
- Poon T. and Ovadia R., (2008), Using tactile learning aids for students with visual impairments in a first semester organic chemistry course, J. Chem. Educ., 85(2), 240–242.
- Poppe K. J., (2008), Azer's interactive periodic table study set, Louisville: American Printing House for the Blind, Inc.
- Repro-Tronics, Inc., http://www.repro-tronics.com/thermo.html, last accessed 15 September 2013.
- Ricker K. and Rogers N., (1981), Modifying instructional materials for use with visually impaired students, Am. Biol. Teach., 43(9), 490–501.
- Samaras A. P., (2011), Self-study teacher research: Improving your practice through collaborative inquiry, Thousand Oaks, California: Sage Publications.
- Sanger M. J., (2005), Evaluating students' conceptual understandings of balanced equations and stoichiometric ratios using a particulate drawing, J. Chem. Educ., 82(1), 131–134.
- Sawrey B., (1990), Concept learning versus problem solving: revisited, J. Chem. Educ., 67(3), 253–254.
- Shane J. W. and Bodner G. M., (2006), General chemistry students' understanding of structure-function relationships, Chem. Educ., 11(2), 130–137.
- Smith D., (1981), Teaching aids for visually handicapped students in introductory chemistry courses, J. Chem. Educ., 58(3), 226–227.
- Smith K. J. and Metz P. A., (1996), Evaluating students understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73(3), 233–235.
- Smothers S. M. and Goldston M. J., (2009), Atoms, elements, molecules, and matter: an investigation into the congenitally blind adolescents' conceptual frameworks on the nature of matter, Sci. Educ., 94(3), 448–477.
- Supalo C., (2005), Techniques to enhance instructors' teaching effectiveness with chemistry students who are blind or visually impaired, J. Chem. Educ., 82(10), 1513–1518.
- Supalo C. A., Mallouk T. E., Amorosi C., Lanouette J., Wohlers H. D. and McEnnis K., (2009), Using adaptive tools and techniques to teach a class of students who are blind or low-vision, J. Chem. Educ., 86(5), 587–591.
- Tallman D. E., (1978), A pH titration apparatus for the blind student, J. Chem. Educ., 55(9), 605–606.
- Tombaugh D., (1981), Chemistry and the visually impaired, J. Chem. Educ., 58(3), 222–226.
- Waldrop J. and Stern S. M., (2003), Disability Status: 2000. Retrieved April 20, 2010, from http://www.ci.osweo.or.us/plan/Land_Use_App/2007/LU%2007-0031/1-5-09%20Exhibits/F-21%20pt.%205.pdf.
- Wild T. and Allen A., (2009), Policy analysis of science-based best practices for students with visual impairments, J. Vis. Impair. Blind., 103(2), 113–117.
- Willoughby D. and Duffy S., (1989), Handbook for itinerant and resource teachers of blind and visually impaired students, Baltimore: National Federation of the Blind.
- Yarroch W. L., (1985), Student understanding of chemical equation balancing, J. Res. Sci. Teach., 22(5), 449–459.
| This journal is © The Royal Society of Chemistry 2013 |