The effect of instructional analogies in interaction with logical thinking ability on achievement and attitude toward chemistry
Fathi-Azar Eskandar*a,
Mansor Bayramib,
Shahram Vahedic and
Vahideh Abdollahi Adli Ansarc
aUniversity of Tabriz, Tabriz 5165663441, Islamic Republic of Iran. E-mail: e-fathiazar@tabrizu.ac.ir
bUniversity of Tabriz – Psychology, Tabriz, East Azerbaijan, Islamic Republic of Iran
cUniversity of Tabriz – Education, Tabriz 0411, Islamic Republic of Iran
First published on 17th July 2013
Abstract
We investigated the effect of instructional analogies in interaction with logical thinking ability on achievement and attitude towards chemistry. The participants were 147 female students from 6 8th grade classes in three public junior high schools selected by using a random multistage sampling method from five education districts in Tabriz, a metropolis northwest of Iran. The classes were randomly assigned to two experimental groups (E1 and E2) and a control group (C), each group consisted of two classes. Group E1 was taught using an analogy model, group E2 received textual elaborated analogy as a supplementary activity, and group C was taught using traditional instruction. The data were collected through a chemistry achievement test, attitude towards chemistry scale, and a logical thinking ability test. The results showed that students with concrete thinking at knowledge level of chemistry achievement test in E2 group had better performance than E1 and C groups and the use of teaching with the analogy model had no significant effect on the students’ chemistry achievement. Also, significant difference was not found between the three study groups in terms of interest and self-confidence as subscales of attitude towards chemistry.
Introduction
Chemistry is one of the subjects that students have difficulties in learning. The concepts in chemistry are abstract in nature and students need to create mental images of the things which cannot be seen. Expert instructors have introduced some appropriate instructional strategies, such as analogy, to facilitate comprehension of abstract concepts.An analogy is a comparison of the similarities of two concepts. The familiar concept is called the analogy and the unfamiliar science concept is called the target (Glynn, 2008, p. 114). Analogies are widely used when explaining ideas, especially in instructional contexts. Using analogies is “one of the core processes of cognition” (Forbus, 2001). They are a key component of learning-by-example and are quite common in science education (Glynn, 1991).
Analogies are double-edged swords: they can foster understanding, but they can also lead to misconceptions (Glynn, 2008). Consequently, the use of analogies in science education as effective instructional tools needs to be presented in a systematic way. One of the well known instructional models in the literature is Glynn’s (1991) teaching-with-analogy model (TWA) which has emerged as being best suited for use in secondary school science classrooms (Haririson, 1992). Instructional analogies are used to teach a variety of subjects including science (e.g., Glynn and Takahashi, 1998; Treagust et al., 1998), and mathematics (Akman, 2005; Sarina and Namukasa, 2010). Analogies are believed to help students learning by providing visualization of abstract concepts, by helping the students to compare similarities from the real world with the new concepts, and by increasing students’ motivation (Duit, 1991). There are contradictory findings around the effect of analogies on students learning. Some studies have indicated that analogies enable complex scientific concepts to be perceived meaningfully, in teaching and learning science (Wong, 1993; Glynn et al., 1996). According to Wong (1993) analogies are dynamic tools which facilitate perception and enable problem-solving. Other researchers (Harrison and Treagust, 1993; Thiele et al., 1995) have stated that analogies are useful for conceptual change in learning science. The study of Yilmaz (2004) revealed that analogy-enhanced instruction accompanied with concept maps caused a significantly better acquisition of scientific conception related to the topic of acid–base and produced significantly higher positive attitudes towards chemistry as a school subject than the traditionally designed chemistry instruction. According to Thiele and Treagust (1991), the analogies help to arrange existing memory and prepare it for new information. However, some studies suggest that using analogies does not have much effect on achievement and perceiving scientific concepts (Drugge and Kass, 1978; Gilbert, 1989). According to Glynn (1991) and Treagust (1993), the use of analogies in a classroom can have a negative effect, even when teachers use a systematic model for teaching with analogies. Although analogy is useful for learning new information, the but information might be superfluous if the student already has an understanding of the target concept being taught (e.g., Venville and Treagust, 1997) and may have no effect on students' performance in achievement tests. Some studies (e.g., Bean et al., 1990; Friedel et al., 1990) have reported that the use of analogies has had little or no effect on learning. There are some factors which limit the efficiency of analogies (Duit, 1991; Thagard, 1992) and they may be the reason of such contradictory findings. One of these factors is the ability of reasoning. There is general agreement that analogies can help students who primarily function at lower cognitive stages. However if these students lack visual imagery and analogical reasoning then the use of analogies is believed to be limited (Gable and Sherwood, 1980). In addition, those students already functioning at a formal operational level may have already attained a sufficient understanding of the target concept and the inclusion of an analogy may add an unnecessary information load resulting in new alternative conceptions being formed by the students (Thiele and Treagust, 1991).
Learning of science requires intellectual skills and high levels of reasoning ability of students (Lawson, 1982; Bitner, 1991); thus, a positive correlation between students' logical thinking ability and their achievement has been reported (Johnson and Lawson, 1998; Jones et al., 2000; Elliot, 2006). For successful learning in science, five formal reasoning modes consisting of controlling variables, proportional, probabilistic, correlational, and combinational reasoning abilities are essential (Bitner, 1991). The results of various studies indicate that the majority of middle and even secondary school students do not reach formal operation levels (Shemesh et al., 1992; Adey and Shayer, 1994). The study of Tobin and Capie (1982) showed that formal reasoning ability is the strongest predictor of process skill achievement and retention with 36% of variance explained. Furthermore, science education researchers have investigated the relationships of analogy effectiveness to Piagetian stages of cognitive development. The literature reveals that analogies are employed most often when the target has a Piagetian formal or abstract nature and the analogy is a concrete stage. Because more science content is beyond the limits of our own senses. For example, chemistry requires the examination of the submicroscopic realm where direct sensory experience is not possible (Thiele and Treagust, 1991). Thus, teaching with analogies may be effective for students with lower cognitive development. The results of studies of Gable and Sherwood (1980) indicated that using analogies may have been effective for students of lower formal reasoning ability but not especially useful for more academically capable students. Students operating at the concrete or transitional stages of development require assistance if abstract or formal cognitions occur. Sarantopoulos and Tsaparlis (2004) reported that analogies can be more effective for students with lower cognitive development.
Students attitudes towards science have long been regarded as one of the most important outcomes of science education (Lin, 1998). George (2006) stated that attitudes towards science and science teaching are issues with longstanding attention and interest in science education research. Hendrickson (1997) claimed that attitudes are the best predictor for estimation of students' success. Sarantopoulos and Tsaparlis (2004) found that analogies, from the affective point of view, have a positive effect on most students. The results of the Yilmaz's study (2004) showed that the use of analogy-enhanced instruction produced a significantly higher positive attitude towards chemistry as a school subject. The majority of the existing studies about attitudes concern attitude towards science and there were few studies about attitude towards a particular subject like chemistry (e.g., Yilmaz, 2004), biology and physics. Thus, there is need for more studies about attitudes towards particular subjects in relation to teaching methods.
Since concept development is a justifiable mean to acquire scientific knowledge and this is true when students have alternative conceptions about a particularly concept, using analogies can assist in concept development. Also, relatively little is known about the influence of logical thinking ability level in analogies. As such, the present study represents a contribution in this regard.
Purpose and research questions
A review of existing literature about analogies indicates that a few studies examined the effectiveness of analogies in relation to different levels of students' logical thinking ability. Therefore, the aim of this study was to investigate the effect of instructional analogy on logical thinking ability in terms of achievement and attitude towards chemistry in the 8th grade of public junior high school. The guiding research questions were:(1) What is the effect of teaching with analogy and textual elaborated analogy as a supplementary activity on students' achievement with different levels of logical thinking ability?
(2) What is the effect of teaching with analogy and textual elaborated analogy as a supplementary activity on students' attitude towards chemistry?
Methods
Participants
The participants were 147 female students from 6 8th grade classes in three public junior high schools selected by using a random multistage sampling method from five education districts in Tabriz, a metropolis northwest of Iran. The organization pattern of formal education in Iran is, 6 years of elementary and 6 years of secondary school (3 years junior high school and 3 years senior high school). Usually, age groups 12 to 18 years old are enrolled in secondary school. In this study, the students' ages ranged from 13 to 14 years old. The students in the 7th grade became familiar with “matter and its changes” and in the 8th grade, they should study issues related to “atomic structure and chemical composition” in regard to the chemistry course.Materials
In this study, the researchers, with the guidance of four science teachers and a chemistry professor, prepared five instructional models including: the Thomson atomic model, Rutherford atomic model, Bohr atomic model, chemical composition, and ionic bonding adapted from Glynn's TWA model. The models included the nucleus, electron orbits, electrons, protons, neutrons, atoms, molecules, covalent bonds, ions and ionic bonding concepts. It should be mentioned that some kinds of analogies are included by the curriculum developer in the textbooks. However, they were not in accordance with Glynn's TWA model. Thus, the textual elaborated analogies were constructed following the guidelines in Glynn's TWA model. The textual elaborated analogies compared verbal and visual features of analogy concept systematically, for example the solar system, to the target concept, the Bohr atomic model. It was expected that the mapping of the analogy concepts features onto the target concepts features would make target concepts features more meaningful and therefore more memorable. Table 1 shows five target concepts and analogies used in the science textbook.| Target concepts | Analogies |
|---|---|
| Thomson atomic model | Raising cake |
| Rutherford atomic model | Football field |
| Bohr atomic model | Solar system |
| Chemical composition | Puzzle pieces |
| Ionic bonding | Magnetic poles |
Research design and procedure
The study employed a quasi-experimental pre- and post-test/control group design. The independent variable was instructional method with three levels (teaching-with analogy model, providing extracurricular textual elaborated analogies and traditional instruction) and the moderator independent variable was logical thinking ability with two levels (concrete thinking and transitional thinking). Achievement with two levels (knowledge and comprehension) and attitude towards chemistry scores with two subscales (interest and self-confidence) were considered as the dependent variables.In one of the public junior high schools, three classes were randomly chosen as the experimental and control groups and the students were taught in three different modes including: teaching-with analogy model (e1, n = 24), providing a textual elaborated analogy as a supplementary activity (e2, n = 25), and traditional instruction (c1, n = 25), by a teacher. In two other public junior high schools, three teachers taught the target concepts to the students in three classes by using the methods of teaching-with analogy (e3, n = 25), providing a textual elaborated analogy as a supplementary activity, (e4, n = 23), and traditional instruction (c2, n = 25). In order to increase the internal validity of the research and control the effect of experimental treatment diffusion and teacher (Gall et al., 2007), the experimental and control groups were combined as E1 (e1 + e3), E2 (e2 + e4), and C (c1 + c2).
In this study, the same models, i.e., the Thomson atomic model, Rutherford atomic model, Bohr atomic model, chemical composition, and ionic bonding, were covered for both the experimental and control groups. The analogies were taken from a science textbook, published by the government, which is popular for all of the students in the 8th grade of public junior high school. In general, students were given equal opportunities to perform the educational activities in each group. The experimental group E1 was taught with analogical instruction by using Glynn's teaching-with-analogy (TWA) model to teach each concept. During the instruction time, the teachers of experimental groups E1 followed the six steps in the mode: (1) introduce the concept to be learned, (2) review the analogy concept, (3) identify the relevant features of the target and analogy, (4) connect (map) similarities between the target and analogy, (5) indicate where the analogy breaks down, and (6) draw conclusions about the concept (Glynn, 2008). During the instruction, some analogies were shown directly to students in the classroom by using the releveant tools, for example: raisin cake, puzzle pieces, and magnetic poles. However, the pictures of other analogies, for example: the solar system and football field, were drawn on the blackboard and presented to the students. During the presentation of the analogies in the classroom, students were assisted to relate the analogy concepts and target concepts by the help of some questions. By this way, the teachers contributed to the maximum participation of students in the lessons. At the end of the presented analogies (after the discussion between students) the teachers explained the similarities and differences between the analogy and target concepts again. Therefore, the students who found an incorrect relation between the analogy and target concepts changed their opinion. In the experimental group E2, the teachers divided the class time into two parts. During the first session, the target concepts were taught using lecture and discussion methods and using the analogies of the science textbook presented in an unsystematic way, i.e., explaining similarities between analogy features and target features without systematic mapping. During the second session, the teachers gave students the same instructional concepts with textual elaborated analogy as a supplementary activity and wanted them to study it carefully and lastly, the teachers asked some questions to ensure that the students had studied textual elaborated analogy or not. Students in group C received traditional instruction which involves teaching the concepts by lecture and discussion sessions following the use of analogies as presented in the science textbook in an unsystematic way. The target concepts were taught in five sessions each lasting 45 minutes. The teachers were informed not to take any written exam before taking a post-test CAT (chemistry achievement test) on the target concepts. The CAT was administered without prior notification after two weeks, in order to achieve accurate information and reduce the effect of instruction of the latest target concept on the post-test scores.
CAT and attitude towards chemistry scale (ACS) were applied as pre-test and post-test. Thus, prior to experimental treatment, pre-test CAT was conducted for 20 minutes and pre-test ACS for 15 minutes in the experimental and control groups, simultaneously. Also, in a separate session, the test of logical thinking ability (TOLT) was administered for 45 minutes on students and they were completely justified about the aim and the way of answering the questions before the test.
Measurements
In addition, to get a score of 1 for items 9 and 10, students need to list all the possible combinations. Applying factor analysis, and correlating the students’ scores in TOLT and their performance in the assignments related to Piaget's traditional interview, Tobin and Capie (1981) have strongly confirmed test validity and have used students’ performance in the tasks of Piaget's traditional interview as a basis to classify the subjects as concrete, transitional, and rigorous formal. On the other hand, TOLT has been used in many studies to indicate test validity (Lawson et al., 2006; Soylu, 2006). Tobin and Capie (1981) have reported a Cronbach's alpha reliability coefficient of the instrument of 0.85 which indicates high internal consistent reliability of test items.
It is worthwhile to mention that TOLT was translated to the Farsi language by the two researchers and a physics professor separately. Then, the Farsi translations were compared and modifications were made. Finally, it was translated to English by an English translator and another physics professor. Comparing the test in the English translation with the original English form, some minor mistakes were corrected; thus, the translation was validated. In a pilot study, to determine the reliability of the test, 124 8th grade students fro a public junior high school in Tabriz city were chosen randomly and the test was administered. Prior to the students responding to the test questions, the researcher explained the purpose, manner and time required for response to the test (45 minutes). Based on the results of this pilot study the reliability coefficient of the test was 0.71.
Prior to analysis of the main components, the suitability of the data for factor analysis was assessed. Inspection of correlation matrix revealed the presence of many coefficients of 0.3 and above. The Kaiser–Meyer–Olkin value was 0.84, exceeding the recommended value of 0.6 and Bartlett's test of sphericity was significant (χ2(171) = 1207.01, p < 0.01), and supporting the factorability of the correlation matrix. According to the results of the initial factor extraction, four components had Eigen values of greater than one and 47.86% of the variance was explained by these four factors. In addition to factor extraction statistics, the screen plot was also used to decide the number of factors that will be extracted. Considering the results, it was decided that extracting two factors is possible. These two factors explained 39.56% of the variance in total. Oblimin rotation was performed to help the interpretation of these two components. The rotated solution revealed the presence of a simple structure, with both components showing a number of strong loadings and all the variables loading substantially on only one component. The pattern and structure matrix with oblimin rotation of two factor solution of ACS items is presented in Table 2.
| Item | Structure coefficients | Pattern coefficients | ||
|---|---|---|---|---|
| Component 1 | Component 2 | Component 1 | Component 2 | |
| 1 | 0.755 | 0.043 | 0.789 | 0.143 |
| 5 | 0.736 | 0.411 | 0.676 | 0.252 |
| 7 | 0.658 | 0.221 | 0.642 | 0.070 |
| 9 | −0.537 | −0.135 | −0.535 | −0.008 |
| 6 | 0.530 | −0.119 | 0.531 | −0.006 |
| 8 | 0.553 | 0.547 | 0.589 | 0.441 |
| 10 | 0.566 | 0.441 | 0.515 | 0.243 |
| 12 | 0.098 | 0.662 | 0.062 | 0.677 |
| 14 | 0.250 | 0.634 | 0.107 | 0.608 |
| 2 | 0.506 | 0.681 | 0.366 | 0.594 |
| 4 | −0.332 | 0.552 | −0.465 | 0.562 |
| 11 | 0.322 | 0.549 | 0.204 | 0.501 |
| 3 | 0.539 | 0.110 | 0.521 | 0.018 |
| 13 | 0.598 | −0.060 | 0.584 | −0.031 |
According to the test purpose, the first component consisting of 9 items was named “interest in chemistry” and the second one consisting of 5 items was determined as self-confidence in learning chemistry. The reliability coefficients for each of the subscales were examined using Cranach's alpha. The alphas were excellent: 0.89 for interest and 0.85 for self-confidence. The ATC scale was used for pre-test and post-test.
Results
In this study, means and standard deviations were calculated for each variable. The data was analyzed using the chi-square test, one-way analysis of variance (ANOVA) and two-way multivariate analysis of variance (MANOVA). Table 3 shows the frequency distribution of the students' logical thinking ability levels in each group and the results of the chi-square test.| Groups | E1 | E2 | C | Total | chi-square test | ||||
|---|---|---|---|---|---|---|---|---|---|
| N.S: no statistically significant at p < 0.05. | |||||||||
| TOLT levels | C | T | C | T | C | T | C | T | X2 = 5.57 df = 2, p = 0.07(N.S) |
| Concrete /transitional | |||||||||
| Frequencies | 41 | 8 | 45 | 5 | 33 | 15 | 119 | 28 | |
| Percentage | 83.67 | 16.33 | 90.0 | 10.0 | 68.75 | 31.25 | 81.0 | 19.0 | |
As is shown in Table 3, the majority of the sample (81.0%) was at the concrete thinking level and a few of them (19.0%) were at the transitional thinking level. In comparison with other groups, a greater percentage of students in group C (31.25%) were at the transitional thinking level. While 16.33% of students in the group E1 and 10.0% of students in group E2 were at the transitional thinking level and the rest of the students, namely 83.67% of students in group E1 and 90.0% of students in group E2 were at the concrete thinking level. The chi-square test was conducted to determine the statistically significant difference among experimental and control groups in pre-test TOLT. The analysis revealed that there was no statistical significant difference in relation to levels of logical thinking ability among the three groups (X2 = 5.57, df = 2, p > 0.05).
The assumptions of one-way ANOVA and two-way MANOVA based on students' scores on the pre-test and post-test were tested. For the normality assumption, as seen from Table 4, the skewness and kurtosis values of the variables fall within the acceptable ranges of (±1), therefore the data is normally distributed (Garson, 2007). Moreover, the other assumptions, the independence of observations and Levene's test of equality of error variances were also tested in order to continue the analysis. A non-significant Box's M indicated that the homogeneity of variance–covariance matrix assumption was not violated.
| Group | Mean | Sd | Skewness | Kurtosis | |
|---|---|---|---|---|---|
| Pre-test knowledge | E1 | 2.32 | 1.2 | 0.46 | −0.81 |
| E2 | 2.54 | 1.14 | 0.02 | −0.36 | |
| C | 2.63 | 1.17 | 0.75 | −0.42 | |
| Pre-test comprehension | E1 | 4.09 | 2.44 | 0.56 | −0.78 |
| E2 | 4.30 | 2.65 | 0.23 | −0.77 | |
| C | 4.66 | 4.66 | 0.34 | −0.99 | |
| Pre-test self-confidence | E1 | 35.40 | 4.11 | −0.25 | −0.86 |
| E2 | 33.76 | 3.34 | 0.29 | −0.25 | |
| C | 35.58 | 4.70 | −0.63 | −0.20 | |
| Pre-test interest | E1 | 18.97 | 5.8 | −0.14 | −0.21 |
| E2 | 18.51 | 4.56 | 0.09 | −0.65 | |
| C | 18.75 | 5.01 | −0.43 | −0.40 | |
| Post-test knowledge | E1 | 3.75 | 1.82 | −0.12 | 0.88 |
| E2 | 3.2 | 1.66 | −0.56 | 0.26 | |
| C | 4.56 | 1.76 | −0.88 | −0.02 | |
| Post-test comprehension | E1 | 9.24 | 3.4 | −0.32 | 0.21 |
| E2 | 7.36 | 2.7 | −0.20 | −0.53 | |
| C | 10.33 | 3.12 | −0.19 | −0.55 | |
| Post-test self-confidence | E1 | 34.95 | 4.2 | −0.43 | −0.92 |
| E2 | 35.23 | 4.80 | −0.12 | −0.48 | |
| C | 36.47 | 4.21 | −0.79 | 0.18 | |
| Post-test interest | E1 | 18.95 | 4.84 | −0.51 | −0.42 |
| E2 | 18.92 | 4.12 | −0.08 | −0.55 | |
| C | 19.22 | 5.05 | −0.93 | 0.38 | |
One-way ANOVA was used to determine differences among groups' mean scores in the chemistry achievement pre-test as appeared in the first research question of the study. The results are shown in Table 5.
| SS | df | MS | F | P | ||
|---|---|---|---|---|---|---|
| N.S: not statistically significant at p < 0.05. | ||||||
| Pre-test knowledge | Between group | 4.70 | 2 | 2.35 | ||
| Within group | 240.21 | 145 | 1.54 | 1.52 | 0.22(N.S) | |
| Total | 244.91 | 147 | ||||
| Pre-test comprehension | Between group | 7.15 | 2 | 3.57 | ||
| Within group | 859.40 | 145 | 5.5 | 0.65 | 0.52(N.S) | |
| Total | 866.56 | 147 | ||||
As is shown from the results presented in Table 5, there was no significant difference between the experimental and control groups in achievement pre-test at the knowledge level (F = 1.52, p > 0.05) and comprehension (F = 0.65, p > 0.05).
A two-way MANOVA was conducted to determine the effect of group and logical thinking ability on chemistry achievement post-test at knowledge and comprehension levels as dependent variables in regard to the first research question of the study. Results from MANOVA are shown in Table 6.
As is shown in Table 6, there was a statistically significant interaction effect between group and logical thinking ability (Pillai's trace = 0.048, F = 3.07, p < 0.05). The multivariate η2 based on Pillai's trace was weak at 0.049. Examination of the univariat effects shows that the interaction effect between the group and logical thinking ability on chemistry achievement post-test at knowledge level was statistically significant (F = 3.07, p = 0.04, η2 = 0.042), but the interaction effect between group and logical thinking ability on chemistry achievement post-test at comprehension level was not significant (F = 1.15, p = 0.22, η2 = 0.021). Results from the ANOVA are reported in Table 7.
The nature of the two-way interaction is depicted in Fig. 1.
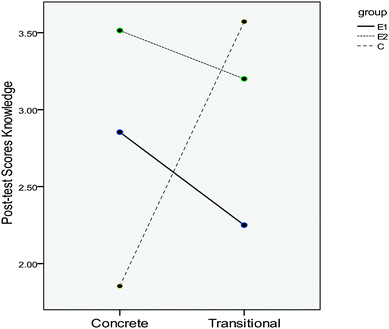 | ||
| Fig. 1 Knowledge scores at the concrete and transitional thinking levels of the experimental and control groups. | ||
As shown in Fig. 1, the mean scores of students at the knowledge level of achievement post-test with concrete thinking in group E2 were higher than the mean scores of students with concrete thinking in groups E1 and C.
Because the interaction between independent variables was significant, we chose to ignore the main effects of the group and logical thinking ability and instead examined the simple main effects of the group on achievement post-test at the knowledge level. The results of one-way analysis of variance indicated that there was significant difference among the experimental and control groups in the concrete thinking level (F(2,116) = 8.87, p < 0.05), but there was no significant difference between the experimental and control groups in the transitional thinking level (F(2,25) = 1.54, p = 0.23). The results are shown in Table 8.
| Concrete thinking | SS | df | MS | F | P | |
|---|---|---|---|---|---|---|
| N.S: not (statistically) significant at the 0.05 level.a The mean difference is significant at the 0.05 level. | ||||||
| Post-test knowledge | Between group | 47.06 | 2 | 23.53 | ||
| Within group | 307.57 | 116 | 2.65 | 8.87 | 0.001a | |
| Total | 354.63 | 118 | ||||
| Post-test comprehension | Between group | 8.26 | 2 | 4.13 | ||
| Within group | 66.70 | 25 | 2.66 | 1.54 | 0.23(N.S) | |
| Total | 74.96 | 27 | ||||
Examination of the pairwise comparisons and means revealed that there was a significant difference between the mean scores of groups E2 and C (p < 0.05) in the achievement post-test at knowledge level, but there was no significant difference between groups E1 and C (p = 0.08) and groups E1 and E2 (p = 0.33) in the achievement post-test at the knowledge level.
One-way ANOVA was used to compare the pre-test mean scores of attitude towards chemistry subscales as appeared in the second research question of the study. The one-way ANOVA results of analysis of attitude towards chemistry pre-test are reported in Table 9.
| SS | df | MS | F | P | ||
|---|---|---|---|---|---|---|
| N.S: not (statistically) significant at the 0.05 level. | ||||||
| Pre-test self-confidence | Between group | 86.00 | 2 | 43.00 | ||
| Within group | 5411.79 | 145 | 37.58 | 1.14 | 0.32(N.S) | |
| Total | 5497.79 | 147 | ||||
| Pre-test interest | Between group | 6.74 | 2 | 3.37 | ||
| Within group | 1068.53 | 145 | 7.42 | 1.37 | 0.25(N.S) | |
| Total | 1075.27 | 147 | ||||
As it appears in Table 9, there wasn’t a significant difference in pre-test mean scores of self-confidence (F = 1.14, p > 0.05) and interest (F = 1.37, p > 0.05) among the experimental and control groups. In the follow up, a one-way MANOVA was conducted to determine the effect of the group on the two dependent variables, post-test mean scores of interest and self-confidence. The results of MANOVA showed that there weren't significant differences among the three study groups on the dependent measures, Wilks' Δ = 0.98, F(4,284) = 0.49, p > 0.05, η2 = 0.007. Since the Wilks' Δ test was not significant, the univariat effects were not examined. The results of the MANOVA are presented in Table 10.
| Effect | Value | F | Hypothesis df | Error df | P | η2 |
|---|---|---|---|---|---|---|
| N.S: not (statistically) significant at the 0.05 level. | ||||||
| Group Wilks' Δ | 0.98 | 0.49 | 4 | 284 | 0.1(N.S) | 0.007 |
Discussion
In this study, the effect of instructional analogies in interaction with logical thinking ability on chemistry achievement and attitude towards chemistry was investigated. The results of pre-test TOLT showed that the majority of students were at the concrete thinking level and only a few students had reached the transitional thinking level, while their ages were 13–14 years. The finding is inconsistent with Piaget's theory of cognitive development in which students reach the formal operational level at 11 or 12 years old. Considering literature, the universality of the first three stages of cognitive development has been substantially confirmed, however the stage of formal operational thinking, has not been confirmed (Lawson, 1995). It is worthwhile to mention that research on Asian students indicates that the majority of secondary school students are at a concrete thinking level (Soylu, 2006; Yenilmez et al., 2006; Baser, 2007). According to Fah (2009) students' low logical thinking abilities may be due to the education system which is more exams-orientated. Hence, less emphasis is given on developing thinking skills. Science teaching and learning strategies are aligned to objectivity with the aim of covering the syllabus within the allotted time without “investing” too much time on nurturing thinking skills among students. Furthermore, school evaluation systems which only emphasize the acquisition of content knowledge contribute to low logical thinking abilities among students.One of the interesting results of the study is that the students at the concrete thinking level who had received extracurricular textual elaborated analogy had better performance in the knowledge test. Such a finding can be explained by pointing out that in group E2 (providing extracurricular textual elaborated analogy) the information of target concepts was more likely to be repeated. Because in addition to the teacher's explanations, students had also received extracurricular textual elaborated analogy. On the other hand, the cognitive development level of students with concrete thinking just enabled them to effectively use the textual elaborated analogy for answering the knowledge test but, was not sufficient to respond to the comprehension test. The role of logical thinking ability in the effectiveness of instructional analogy has very little empirical support. Therefore, more studies are needed to support the results of this research further.
The results of the study also indicated that the use of teaching with analogy models had no significant effect on students' chemistry achievement. Some studies have reported similar findings, for example, Bean et al. (1990) have reported that the use of analogies had no effect on learning. In explaining this finding, Gable and Sherwood's (1980) remarks can be cited. Thus, if students who primarily function at lower cognitive stages lack visual imagery, analogies reasoning, or correlational reasoning, then the use of analogies is still believed to be limited. Since most of the students in this study were at the concrete thinking level, teaching with an analogy model had no significant effect on the students' chemistry achievement.
The results revealed that use of teaching with analogy models and providing textual elaborated analogies as a supplementary activity is not effective for the students' interest and self-confidence as subscales of attitude towards chemistry. To explore the finding, it can be said that science teachers frequently preface their explanation with expressions, such as, “it's just like,” “similarity,” and “likewise”. These recommended expressions would be telling the students “let me give you an analogy” (Glynn, 2008). According to these explanations, the teachers of the control group also used analogy in non- systematic ways to explain the concepts of chemistry while the teachers of the experimental groups used analogies in systematic ways (systematic comparison, verbally or visually, between the features of analogy and target). Thus, probably, teaching the same concepts in the analogy model could not have sparked the interest of the students of the experimental groups toward the chemistry lesson itself. On the other hand, the low cognitive development of students has hindered them from taking advantage of systematic comparisons and getting better marks in the chemistry achievement test, at the level of knowledge and comprehension. Therefore, using instructional analogies has not caused changes to the students' self-confidence and in general, to their attitudes towards chemistry. Also, it should be noted that in previous research investigations were mostly on the use of analogies in teaching and learning, not in chemistry or science (e.g., Sarantopoulos and Tsaparlis, 2004). In these studies students had often reported a positive attitude to the use of analogies in instructional not their attitude towards chemistry. Therefore, to realize the effect of instructional analogies on attitude towards scientific subjects further conclusive research needs to be carried out.
Conclusion and recommendation
In this study, the role of instructional analogies in interaction with logical thinking ability was examined for students' chemistry achievements. When the teachers used textbook analogies, applying different teaching methods, such as teaching with analogy model and traditional instruction, had the same relative impact effect on the achievement test scores of the students. But taking into account the different levels of logical thinking ability, the students with concrete thinking had taken more advantage of the textual elaborated analogy as a supplementary activity. It should be noted that the findings regarding the effectiveness of teaching with the analogies model is where the teachers explain analogies of the science textbook systematically namely, adjusting teaching-with analogy model, and in the future, more investigations are needed to find out the efficacy of teaching with analogy models in comparison with traditional instructions in situations where teachers use self-generated or any other analogies for science concepts.One of the limitations of this study concerns the familiarity rate of students with textbook analogy concepts. Although all the students stated that they are familiar with analogy concepts in science textbooks, it is likely that some were less familiar than others. Random assignment conditions controlled for this variable; however, it would be useful in future studies to precisely assess the students' familiarity with the analogy by means of a rating scale. The degree of familiarity could then be taken into account when analyzing and interpreting data. Another limitation is that a self-reported scale for the purposes of comparing different groups' attitude towards chemistry was used. This study would have been enhanced if an interview had been conducted during the study. Use of interviewing, could gain more accurate and precise understanding about students' attitude. Finally, the subjects of this study were female students thus the results can not be generalized for male students.
Appendix I
Sample analogy used in group E1
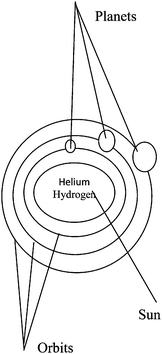
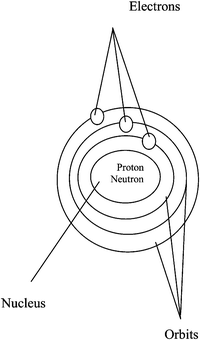
An analogy is found between the Bohr atomic structure and the solar system.| Solar system | Structure of the atom |
|---|---|
| 1. The sun | A nucleus |
| 2. Planets orbit | Electrons orbit |
| 3. Planets | Electrons |
| 4. The spherical shape of the sun and planets | The spherical shape of nucleus and electrons |
| 5. Fixed distance from the sun to the planets | Fixed distance from the nucleus to the electrons |
| 6. Helium and hydrogen as components of the sun | Proton and neutron as components of the nucleus |
 |
 |
Sample of extracurricular textual elaborated analogy used in group E2
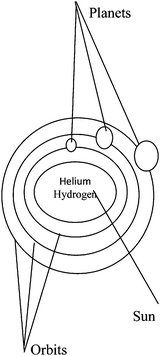
You might think of the Bohr atomic structure as the solar system which is composed of a number of planets. Each planet orbits the sun at a certain distance. Here are some similarities between the solar system and the structure of the atom:| Solar system | Structure of the atom |
|---|---|
| 1. The sun | A nucleus |
| 2. Planets orbit | Electrons orbits |
| 3. Planets | Electrons |
| 4. The spherical shape of the sun and planets | The spherical shape of nucleus and electrons |
| 5. Fixed distance from the sun to the planets | Fixed distance from the nucleus to the electrons |
| 6. Helium and hydrogen as components of the sun | Proton and neutron as components of the nucleus |
 |
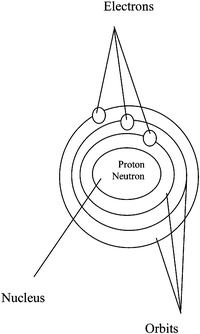 |
Think carefully about each of the above similarities and study the illustration. But remember that this “solar system – the structure of the atom” analogy, like all analogies, breaks down in places. For example, the sun is hot, but a nucleus is not; protons are positive and electrons are negative, whereas the planets and the sun have no charge; the planets are different in size, but electrons are the same size. In general, however, if you remember how the structure of the atom is like the solar system, it will be easier for you to remember the structure of the atom.
Appendix II
Sample of multiple-choice questions based on Bloom's taxonomy (knowledge and comprehension)
1. Which of the following particles are orbiting in circuits around the nucleus? (comprehension)(a) protons, (b) electrons, (c) neutrons, (d) protons and electrons
2. What is the main similarity between the Bohr atomic model and the Rutherford atomic model? (comprehension)
(a) Atoms have no internal structure
(b) Negative charges are concentrated in the nucleus
(c) Positive charges are concentrated in the nucleus
(d) The electrons of an atom are located at specific locations
3. Which of the following particles are held together by covalent bonds? (knowledge)
(a) ions, (b) molecules, (c) atoms, (d) protons
4. Which of the following chemical formula had the ionic bonding? (comprehension)
(a) CH4 (b) CO2 (c) H2O (d) Nacl
5. Which one of the atom particles has no electric charge? (knowledge)
(a) neutrons, (b) electrons, (c) protons, (d) protons and neutrons
References
- Adey P. S. and Shayer M., (1994), Really raising standards: cognitive intervention and academic achievement, London: Routledge.
- Akman C., (2005), The effects of instruction with analogy – enhanced model on ninth grade students' function achievement and attitudes toward mathematics, MA thesis, Perth: Curtin University, available at: http://e etd.lib.metu.edu.tr/upload/12606581/index, accessed 17 April 2011.
- Backhoff E., Larrazolo N. and Rosas M., (2000), The level of difficulty and discrimination power of the Basic Knowledge and Skills Examination (EXHCOBA), available at: http://redie.uabc.mx/vol2no1/contents-backhoff.html, accessed 19 February 2011.
- Baser M., (2007), The contribution of learning motivation, reasoning ability and learning orientation to ninth grade international program students’ understanding of mitosis and meiosis, MA thesis, Ankara, Turkey: Middle East Technical University, available at http://etd.lib.metu.edu.tr/upload/12608544/index.pdf, accessed 21 March 2010.
- Bean T. W., Searles D. and Cowen S., (1990), Text-based analogies, Read. Psychol., 11, 323–333.
- Bitner B. L., (1991), Formal operational reasoning modes: predictors of critical thinking abilities and grades assigned by teachers in science and mathematics for students in grades nine through twelve, J. Res. Sci. Teach., 28, 275–285.
- Drugge N. L. and Kass H., (1978), The effect of selected analogies on understanding of scientific explanations, Abstract of presented papers, NARST, Cleveland, OH: ERIC Clearinghouse for Science, Mathematics, and Environmental Education, The Ohio State University.
- Duit R., (1991), On the role of analogies and metaphors in learning science, Sci. Educ., 75, 649–672.
- Ebel R. L. and Frisbie D. A., (1986), Essentials of education measurement, Englewood Cliffs, NJ: Prentice Hall.
- Elliot S. C., (2006), Is formal reasoning ability related to success on the AP Physics B exam? MA thesis, Faculty of California State University, Fullerton, Dissertation Abstracts International, UMI No, 1433844.
- Fah L. Y., (2009), Logical thinking abilities among form 4 students in the interior division of Sabah, Malaysia, J. Sci. Math. Educ., 32(2), 161–187, available at http://www.wjeis.org/FileUpload/ds217232/File/.1complete.pdf, accessed 17 November 2011.
- Fennema E. and Sherman J., (1976), Fennema-Sherman mathematics attitudes scales: Instruments designed to measure attitudes towards the learning of mathematics by females and males, J. Res. Math. Educ., 7, 324–326.
- Forbus K., (2001), Exploring analogy in the Large, The Analogical Mind: Perspectives from Cognitive Science, Cambridge, MA: MIT Press.
- Friedel A. W., Gabel D. L. and Samuel J., (1990), Using analogs for chemistry solving: does it increase understanding? Sch. Sci. Math., 90, 674–682.
- Gable D. L. and Sherwood R. D., (1980), Effect of using analogies on chemistry achievement according to piagetion level, Sci. Educ., 64, 709–716.
- Gall M. D., Gall J. P. and Borg W. R., (2007), Educational research: an introduction, Boston: Pearson Education.
- Garson D., (2007), Testing of assumptions, key concepts, and terms, available at http://www2.chass.ncsu.edu/garson/pa765/assumpt.htm, accessed 17 December 2012.
- George R., (2006), A cross-domain analysis of change in students' attitudes toward science and attitudes about the utility of science, Int. J. Sci. Educ., 28, 571–589.
- Gilbert S. W., (1989), An evaluation of the use of analogy, simile, and metaphor in science texts, J. Res. Sci. Teach., 26, 315–327.
- Glynn S. M. and Takahashi T., (1998), Learning from analogy-enhanced science text, J. Res. Sci. Teach., 35, 1129–1149.
- Glynn S. M., (1991), Explaining science concepts: a teaching-with-analogies model, in Glynn S., Yeany R. and Britton B. (ed.), The Psychology of Learning Science, New Jersey: Erlbaum, pp. 219–240.
- Glynn S., Law M., Gibson N. and Hawkins C., (1996), Teaching science with analogies: a research for teachers and text book authors, Available at http// currv.edschool.virginia.edu/go/clic/nrrc/scin html, accessed 25 October 2012.
- Glynn S. M., (2008), Making science concepts meaningful to students: teaching with analogies, in Mikelskis-Seifert S., Ringelband U. and Brückmann M. (ed.), Four decades of research in science education: from curriculum development to quality improvement. Münster, Germany: Waxman, pp. 113–125.
- Harrison A. G., (1992), Evaluation of model for teaching analogies in secondary science, MA thesis, Perth, Western Australia: Curtin University, available at http://espace.library.curtin.edu.au/dtl_publish/gen01-era02/22/11315.html, accessed 8 November 2011.
- Harrison A. G. and Treagust D. F., (1993), Teaching with analogies: A case study in grade-10 optics, J. Res. Sci. Teach., 30, 1291–1307.
- Hendrickson A. B., (1997), Predicting student success with the learning and study strategies 14 inventory (LASSI), MA thesis, Iowa State University.
- Johnson M. A. and Lawson A. E., (1998), What are the relative effects of reasoning ability and prior knowledge on biology achievement in expository and inquiry classes? J. Res. Sci. Teach., 35(1), 89–103.
- Jones M. G., Carter G. and Rua M. J., (2000), Exploring the development of conceptual change ecologies: communities of concepts related to convection and heat, J. Res. Sci. Teach., 37, 139–159.
- Lawson A.E., (1982), Formal reasoning, achievement, and intelligence: an issue of importance, Sci. Educ., 66, 77–83.
- Lawson A. E., (1995), Science teaching and the development of thinking, USA: Library of Congress Cataloging-in-Publication Data.
- Lawson A. E., Banks D. L. and Logvin M., (2006), Self-efficacy, reasoning ability, and achievement in college biology, J. Res. Sci. Teach., 44, 706–724.
- Lin H. S., (1998), Enhancing college students' attitudes toward science through the history of science, Proc. Natl. Sci. Counc., Repub. China, Part D, 8, 86–91.
- Salta K. and Tzougraki C., (2004), Attitudes toward chemistry among 11th grade students in high schools in Greece, Sci. Educ., 88(4), 535–547.
- Sarantopoulos P. and Tsaparlis G., (2004), Analogies in chemistry teaching as a means of attainment of cognitive and affective objectives: a longitudinal study in a naturalistic setting, using analogies with a strong social content, Chem. Educ. Res. Pract., 5, 33–50.
- Sarina V. K. and Namukasa I., (2010), Non-math analogies in teaching mathematics, Proc. Soc. Behav. Sci., 2, 5738–5743.
- Shemesh M., Eckstein S. F. and Lazarowitz R., (1992), An experimental study of the development of formal reasoning among secondary school students, Sch. Sci. Math., 92, 26–30.
- Soylu H., (2006), The effect of gender and reasoning ability on the students' understanding of ecological concepts and attitude towards science, MA thesis, Ankara: Middle East Technical University, available at http://www.genderandscience.org/doc/CReport_Turkey.pdf, accessed 11 May 2011.
- Thagard P., (1992), Analogy, explanation, and education, J. Res. Sci. Teach., 29, 557–544.
- Thiele R. B., Venville G. and Treagust D. F., (1995), A comparative analysis of analogies in secondary biology and chemistry textbook used in Australian schools, Res. Sci. Educ., 25, 221–230.
- Thiele R. and Treagust D. F., (1991), Using analogies in secondary chemistry teaching, Aust. Sci. Teach. J., 37, 10–14.
- Tobin K. G. and Capie W., (1981), The development and validation of a group test of logical thinking, Educ. Psychol. Meas., 41, 413–423.
- Tobin K. G. and Capie W., (1982), Relationships between formal reasoning ability, locus of control, academic engagement and integrated process skill achievement, J. Res. Sci. Teach., 19, 113–121.
- Treagust D. F., Harrison A. G. and Venville G. J., (1998), Teaching science effectively with analogies: an approach for pre-service and in-service teacher education, J. Sci. Teach. Educ., 9, 85–101.
- Treagust D. F., (1993), The evolution of an approach for using analogies in teaching and learning science, Res. Sci. Educ., 23, 293–301.
- Venville G. J. and Treagust D. F., (1997), Analogies in biology education: a contentious issue, Am. Biol. Teach., 59, 282–287.
- Wittrock M. C., (1992), Knowledge acquisition and comprehension, in Alkin M. C. (ed.) Encyclopedia of Educational Research, 6th edn, New York: McMillan, pp. 644–705.
- Wong D.E., (1993), Self-generated analogies as a tool for constructing and evaluating explanations of scientific phenomena, J. Res. Sci. Teach., 30, 367–380.
- Yenilmez A., Sungur S. and Tekkaya C., (2006), Students' achievement in relation to reasoning ability, prior knowledge and gender, Res. Sci. Technol. Educ., 24, 129–138.
- Yilmaz O. C., (2004), Effect of analogy – enhanced instruction accompanied with concept maps on understanding of Acid-Base concept, MA thesis, Ankara, Turkey: Middle East Technical University, available at: http://etd.lib.metu.edu.tr/upload/12605247/index.pdf, accessed 15 May 2012.
| This journal is © The Royal Society of Chemistry 2013 |
