Effects of an intellectually caring model on urban African American alternative high school students' conceptual change and achievement in chemistry
Lynda C. Wooda,
Jazlin Ebenezer*b and
Relena Boonea
aSouthfield Public Schools, 24661 Lahser Road, Southfield, Michigan 48033, USA. E-mail: Lcw4766@att.net; BoonerD@southfield.k12.mi.us
bTeacher Education Division, Wayne State University, Detroit, Michigan 48202, USA. E-mail: Aj9570@wayne.edu
First published on 22nd April 2013
Abstract
The purpose of this article is to study the effects of an intellectually caring model of teaching and learning on alternative African American high school students' conceptual change and achievement in a chemistry unit on acids and bases. A mixed-methods approach using retrospective data was utilized. Data secured from the teacher were the audio-recordings of her prior- and post-interventional individual interviews with students and the results of the students' pre- and post-interventional Acid Base Achievement Test (ABA-T). All audio-recorded interviews were transcribed. A qualitative analysis of students' prior- and post-interventional conceptions of acids and bases using phenomenography revealed: (a) a change in the number of categories of descriptions, (b) a shift in language use from everyday talk to more chemical talk, and (c) a hierarchy of chemical knowledge. The ABA-T results indicated that students (n = 17) in the experimental group achieved significantly higher scores (p < 0.003) than students in the control group (n = 22) taught by traditional teaching methods. The study outlines three implications: (a) reaching the often unreached mind, (b) developing simple chemical phrases into coherent chemical explanation, and (c) achieving alternative students' success in traditional test. The study recommends implementing an intellectually caring model for teaching alternative education students.
Introduction
Reggie, a high-school student, was referred to Columbus Charter School by his mentor at Big Brothers/Big Sisters when Reggie was 15. During his initial interview, Reggie got up and walked out. At that point in his life, Reggie clearly was not ready to attend Columbus Charter School. Reggie returned to Columbus the following year and decided to enroll in the programme. Although he attended some classes, his attendance was sporadic, and he dropped out after a few weeks. The following year, Reggie returned to Columbus again. The staff members at Columbus allowed him to enroll but insisted he sign a contract agreeing to remain in the programme. His attendance was irregular, and his academic progress slow. He remained quiet and angry, but this time he stayed. Finally, after several months in the programme, he began to talk more openly with the counselors on staff. He told them about his family – one brother had served seven years in prison, and the other brother was unemployed. The staff called Reggie on the days when he failed to attend school and began checking in with him regarding his experiences at school and other aspects of his daily life. It worked. Something clicked. Reggie's attendance improved, he made progress in his classes, and he began to enjoy his schoolwork. His demeanor and outlook changed dramatically, and he earned a great deal of respect from his peers, as well as his teachers. At the Columbus awards luncheon, he was recognized for having been voted the “Most Improved Student.” He even gave the commencement address at his graduation from Columbus. After Reggie left Columbus, he entered college at Columbus State University. In the spring, he was selected as a member of a university exchange programme to spend six weeks in London (Adapted from Stories of Transformation, Youth Build USA).Reggie is a unique individual, but his circumstances are not. Reggie represents many troubled youth in the urban neighborhoods of the United States who attend comprehensive high schools. Often, students lose hope in the school system and find “life on the streets” a more promising option. We seem to know more about the characteristics of such students than we know about effective instructional practices that will support them from dropping out (Christenson et al., 2001).
According to Becker and Luthar (2002), pedagogical practices in many alternative schools emphasize lower-order skills, such as rote memorization. In fact, classroom observations have identified a frequent reliance on teacher-directed activities, including independent seatwork, rote learning, as well as frequent interruptions of learning activities to manage classroom behavior (Haberman, 1991). Such instructional practices appear to have profound ill-effects on students' motivation to learn, their overall learning experience, and academic success (Darling-Hammond, 2000).
Students who cannot handle normal school learning experiences and fail miserably most frequently have been placed in alternative high schools with the hope that teachers in these alternative high schools may positively impact students' learning and achievement (O'Connor and McCartney, 2007). However, the question remains: How do teachers provide high quality, relevant science education to students – specifically, African–American students in urban secondary schools – who cannot cope with the expectations of comprehensive schools? One answer to this question can be found in the 1996 report by the National Commission on Teaching and America's Future (NCTAF): “What teachers know and can do is the most important influence on what students learn” (p. 34). Teachers knowing and being able to connect the culture of students and the culture of science as well as holding students to high expectations are primary influences of what and how students learn (Mutegi, 2010). In fact, high-quality teaching has been considered the single most important factor influencing achievement gains (Sanders and Horn, 1994). Implementing caring pedagogical practices will also promote student engagement, active learning, motivation to learn, emotional stability, and success in academic work (Gay, 2010). These characteristics suggest that more effective learning outcomes and science achievement might be possible if teachers had a better understanding of learning models that care for the diverse experiences students bring to the learning environment.
The Common Knowledge Construction Model (Ebenezer and Conner, 1998; Ebenezer and Haggerty, 1999; Ebenezer et al., 2010) might be an intellectually caring model for improving alternative high school students' learning because it is rooted in variation theory of learning (also called phenomenography) (Marton and Booth, 1997; Marton and Tsui, 2004). Learning involves a qualitatively different approach to understanding a phenomenon. The variations result in “relational conceptual change” (Ebenezer and Gaskell, 1995, p. 1). Naïve conceptions do not serve a purpose in conceptual change because conceptual change is the appropriation of intellectual tools (Ivarsson et al., 2002).
In contrast, Posner et al. (1982) have proposed four theoretical conditions for conceptual change to occur: “dissatisfaction, intelligible, plausible, and fruitful” (p. 211). Chi and Roscoe (2002) have defined “conceptual change” as the process of repairing misconceptions. These authors have defined “conceptual reorganization, revision and accommodation” as the ongoing development of preconceptions. Pintrich et al. (1993) have referred to this conceptual change model as “cold” and instead proposed a “hot” model of conceptual change that takes into consideration the aspect of student motivation. Van Manen (2002) has highlighted that the conceptual change models do not help in minority students' learning. This is because the traditional conceptual change models call for a confrontational approach to teaching science and requires students to move from their everyday space of thinking to an unfamiliar territory. However, this article argues that the Common Knowledge Construction Model (CKCM – hereafter the model will be referred to CKCM), rooted in an alternative conceptual change theory of learning and built on the principle of caring, reaches the heart and soul of learners because the variation theory of learning advocates the teacher super conscious use of students' conceptions as important frameworks to develop learning experiences.
Using the variation theory of learning, this study was designed to help better understand the issues surrounding alternative education students' relational conceptual change and achievement during a unit on acids and bases. A theoretical foundation to this problem of study is a review of the literature focusing on the following areas: conceptions of teacher pedagogical caring and the intellectually caring CKCM (Ebenezer et al., 2010). Literature review of students' variations of acids and bases and student science achievement introduce the research objectives and questions.
Theoretical frameworks
Conceptions of teacher pedagogical caring
Littky (2004) has reinforced the notion that caring for all students and the need to be cared for are essential components of any learning environment. Noddings (2005) also has supported the idea that when students believe teachers care about them, they are more willing to participate in classroom experiences within a social environment, which engages them in dialogues that lead to negotiating mutual understanding and adopting individual perspectives. Gay (2010) has suggested that students who are engaged in school develop a desire to learn, maintain emotional stability, and succeed in their academic work. If Gay's assertion is accurate, then it is likely that implementing caring practices that support improved relationships within schools and classrooms can promote student engagement. When children do not experience caring from adults in school or at home, it negatively influences their desire and motivation to achieve. Many rebel and many fail academically and behaviorally and are placed in classes or schools designed to address their deviant behavior or failure to succeed academically.McCroskey (1992) has pointed out that it is not simply a matter of caring that counts; rather, it is the perception of caring that is critical. When teachers care deeply, McCroskey has suggested that they naturally communicate that attribute to students. McCroskey has highlighted three key factors that lead students to perceive that teachers care about their well-being: empathy, understanding, and responsiveness. Empathy has been defined as the teachers' capacity to experience situations from students' perspectives and experience how they feel about those situations. Understanding has been defined as the teachers' ability to comprehend students' ideas, feelings, and needs. Responsiveness has been defined as paying attention to students' problems by carefully listening to what they say and responding to their needs without delay. When teachers are empathetic, understanding, and responsive, students perceive them to be caring and attribute to them more credibility. The more that students perceive their teachers care about them, the more likely the students are to care about the class, the more likely they are to care about the course content, and the more likely they are to pay attention in class and consequently demonstrate expected learning. The relationship between caring and teaching has most often been described as “pedagogical caring” (Hull, 1997). Noddings (2005) has argued that educators should aim to develop competent, caring, loving, and lovable dispositions as a moral priority in teaching. Such pedagogical caring might be accommodated by the CKCM, a relational conceptual change model that promotes intellectual empathy in a teaching and learning environment (Ebenezer et al., 2010).
While the goal of curriculum design and pedagogy based on the CKCM is to emulate inquiry practices and processes of the scientific community, the burden of reaching all students in learning science with care is an even greater goal. The foregoing goal has been established to reach those students of science who often have been neglected. Caring, demonstrated in the classroom, calls for a learning environment that accommodates conditions, contexts, activities, and structures that promote, nurture, and support conceptual understanding through inquiry practices among students. Such practices promote a learning community with which students can identify (National Research Council, 2002; Noam et al., 2003; Honig and McDonald, 2005).
Intellectually caring model
The CKCM consists of four interactive phases of teaching and learning as represented by Fig. 1: (1) exploring and categorizing, (2) constructing and negotiating, (3) translating and extending, and (4) reflecting and assessing. In discussing each of these four phases, we explicitly depict how the hallmarks of the variation theory of learning that underpins the CKCM reveals the various aspects of pedagogical caring pointed out in the previous section.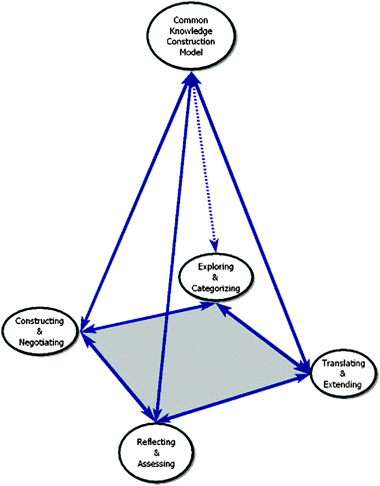 | ||
| Fig. 1 Common knowledge construction model (Ebenezer et al., 2010). | ||
In the exploring and categorizing phase, there is no strong concern for the intellectual capacity or developmental mechanism that created that variability. Images of the world are not present in individual consciousness, but they are reflected in the way we organize society (Marton, 1984, p. 45). Learners' conceptions of reality are particular-to-particular context and problem raised within that context (Saljo, 1988). Thus, phenomenography may be used as an inquiry tool to generate conceptions of a natural phenomenon within a particular context.
To explore students' conceptions of acids–bases, for example, they may be shown a picture of a factory with gases coming out of the smokestacks while it is also raining. The teacher asks “second-order” questions based on the following scenario: What sense do you make of this picture? Can you see what is happening? More complex questions might be as follows: What do you think happens when gases and water mix? How might the combination of gas and water affect the environment? When emotional connections are made to an environmental issue through an activity, students have the opportunity to demonstrate caring practices and value what they are learning. To simulate scientific practice, students are encouraged to explore multiple ideas because the world itself is multifaceted and open to variation in interpretation. In doing so, students begin to understand that science is an attempt to explore and explain natural phenomena in qualitatively different ways. Students' conceptions are interpreted with much intellectual empathy, not judged as correct or incorrect, naïve or misconceptions as would occur in a diagnostic or deficit model (Duit and Treagust, 2003).
Found in the pool of students' expressions are personal conceptions with inter- and intra-variations. The researcher identifies and develops commonalities in meanings into “phenomenographic categories” or the “outcome space” (Marton and Booth, 1997; Marton and Tsui, 2004). The categories of description are ways of denoting the researcher's interpretations of students' conceptions of a phenomenon, not from his or her perspective but from the perspective of the learner (Orgill, 2002). Categories of description consist of qualitative and quantitative aspects. The qualitative aspects are the categories of description, while the quantitative aspect is the frequency distribution related to the categories (Renstrom, 1988).
According to Ebenezer et al. (2010), exploring students' relational conceptions of a science phenomenon with tasks that represent their experience, interpreting their intra- and inter-variations of those conceptions, and categorizing those conceptions with intellectual empathy not as naive or misconceptions, and developing a lesson sequence incorporating students' conceptions as frameworks symbolize “pedagogical care” (Hull, 1997). Thus, in phase one, an attempt is made to reach all students, including the majority/minority, privileged/under privileged, culturally same/different, rich/poor, and regular/alternative students within the dynamics of urban education. Caring for all students and the need to be cared for are human needs (Littky, 2004). It is not simply a matter of caring that counts; rather, it is the perception of caring that is critical (McCroskey, 1992; McCroskey and Teven, 1997). Students perceive teacher caring when teachers demonstrate a genuine capacity to comprehend how students experience, conceptualize, and view in qualitative different ways. Students enter into the construction and negotiation phase with the confidence that their teachers care for them and value their conceptions.
Students' engagement in scientific knowledge construction and negotiation calls for a caring learning environment for all students, particularly those who are disadvantaged. Gay (2010) observes that teacher care for students, who they are and how they perform, will create a desire to learn and succeed academically. When teacher care is manifested, students' self-esteem and confidence eroded over time resulting in a lack of academic success in traditional schools may now improve. By maintaining high expectations and simultaneously demonstrating caring practices, students will know that they and their ideas are valued. Students will clearly perceive that the teacher cares and supports their intellectual development through conceptual change learning.
Because of the phase two type of learning experience, students recognize that conceptual change occurs when they question their original conceptions and submit their ideas to critical thinking processes, inquiry, and peer review. Students also realize that collaborative time and effort are required as well as empathy towards fellow learners when formulating scientific ideas. Through this process, students learn to distinguish their language use in the everyday context and the science classroom context.
As teachers monitor their students' conceptual change learning, they will gain valuable insights about their ideas and their level of understanding. Based on their sense-making of students' evolving conceptions and understandings, teachers will be able to develop meaningful and targeted lessons, and continuously adjust the lesson sequence for future instruction. The teacher enacting the phase two of the CKCM will increase competence to support students through conceptual change inquiry learning with pedagogical care.
In this phase, students are nurtured to develop a critical-thinking disposition through scientific inquiry and problem solving. Personal responsibility from students is elicited via a reflective process based on their values. The types of concerns and issues they value and for which they will be responsible and reflect upon will emerge as a result of the caring environment and meaningful discourse (Noddings, 1992). Students must also perceive that the responsibility they are demonstrating is acknowledged and that their insights are understood, shared, and valued supports a social, intellectual, and ethical progression from (a) self-centeredness to (b) ethical partnerships to (c) ethical caring/support to (d) ethical decision making at a global level. Through school-community partnerships, all students can experience an ethically caring environment that enables them to make intellectual decisions and take action in community affairs (Ebenezer et al., 2010). These authors believe that by ushering deprived students into the scientific community of practice through community partners, they are pointed to STEM higher learning and STEM careers. This initiation into the scientific community to prepare for the future is also an aspect of caring.
Caring is manifested in this phase when students engage in experiences that confirm what they know and, perhaps just as importantly, how they know a concept. Noddings (2010) has suggested that teachers should care about not only the knowledge goals for which students are striving but also the ways that students go about achieving these goals. The use of formative assessments is one way that teachers and students can measure continuous and reflective learning. Encouraging and confirming as highlighted by Noddings (1992) is an integral part of the assessment process.
Students' challenges and conceptions in acids and bases
The conceptual understanding of the topic of acids and bases and related concepts such as pH and neutralization have been challenging for most students (Zoller, 1990; Nakhlen and Krajcik, 1993; Demircioglu et al., 2005). Like other areas in chemistry, it is also important not to neglect the study of acids and bases because chemistry consists of complex networks of ideas. For example, the concepts of acids and bases are connected to the nature of matter, stoichiometry, solutions, and chemical reactions. When the understanding of certain chemical concepts such as acids and bases is clouded, it has the potential to adversely affect the study of related areas (Garnett and Treagust, 1990; Garnett, 1996; Boo and Watson, 2001; Acar and Tarhan, 2007).Concerning challenges in the study of acids and bases, and Ross and Munby (1991) have independently found that students: know more about acids than bases; consider pH as a measurement of the degree of acidity, hold inadequate conceptions of phenomena such as heat being released in an acid base reaction; and have difficulty understanding the ionic nature of acids and bases. Focusing on students' conceptions, Hand and Treagust (1991) identified 60 16-year-old students' qualitatively different conceptions of acids and bases: (a) an acid is something which eats material away and burn you, (b) testing an acid can only be accomplished by using it in an attempt to “eat something away,” (c) to neutralize is to break down an acid or to change from an acid, (d) a base is something which makes up an acid and (e) a strong acid can eat material away faster than a weak acid. The majority of these conceptions are related to acids. With these and other conceptions found in the literature, Peterson et al. (1989) and Treagust (1988) developed the Concept Achievement Test (CAT). Using the CAT, Demircioglu et al. (2005) indicated that the students in the experimental group, taught with the new teaching material in a unit on acids and bases during a four-week trial period, showed significantly greater achievement and reduction in the frequency of students holding prior alternative conceptions compared to the control group. Driver et al. (1994) have identified current media and sensory experiences, including tasting sour foods, watching advertisements, and viewing crime stories and news about acid rain as possible reasons for students' alternative conceptions of acids and bases.
Problem statement
Of the studies presented related to acids and bases, only Demircioglu et al. (2005) and Hand and Treagust (1991) have addressed student conceptions of acids and bases. They used a concept achievement test (CAT) to explore the degree of conceptual change students underwent during a unit on acids and bases. In contrast to prior studies that used the CAT, this study uses phenomenography and variation theory of learning to identify students' conceptions and conceptual changes related to acids and bases. However, both types of studies (CAT and phenomenography) are beginning to have a positive impact on student science achievement (Sungur et al., 2001; Eryilmaz, 2002; Demircioglu et al., 2005; Ebenezer et al., 2010). In response, it is helpful to link the CKCM to students' conceptual changes and their achievement in the topic of acids and bases. Similar to prior conceptual change studies that revealed an impact on science achievement, this study aims to explore the impact of a caring conceptual change inquiry model on students' achievement. Based on these two research goals – that is, conceptual change and its effect on achievement – two related questions are formulated:1. What conceptual changes are evident for a group of urban African–American alternative high school students when immersed in the CKCM-based acid–base lesson sequence?
2. Does the CKCM acid–base lesson sequence significantly improve urban African–American alternative students' achievement compared to (a) prior- and post-interventional teaching and (b) traditional teaching?
This interventional study uses both qualitative and quantitative methods to study the effect of an intellectually caring model of teaching and learning on conceptual change and achievement of alternative students during the enactment of an acid–base lesson sequence. An in-depth analysis using both methods provides confidence in the CKCM that it can serve the intellectual needs of alternative students. Although the reform-based CKCM has been studied in three middle school classrooms-urban (Biernacka and Ebenezer, 2007), affluent (Ebenezer and Puvirajah, 2005), and diverse (Ebenezer et al., 2010), this is the first time it has been used in an urban African–American alternative high school classroom. On account of these research studies in diverse classrooms, the CKCM's practical effectiveness is gaining ground. The CKCM known for intellectual empathy has been for the first time theoretically defended with literature on conceptual caring in order to use it with the alternative high school students. Thus, the results of this study may be helpful in assisting other teachers and researchers who are looking for a caring teaching and learning model to be used with alternative high school students.
Methods
Northwood Scholars Academy
Students attend the Northwood Scholars Academy (NSA), an alternative high school in Northwood Public School, because they are behind academically and have failed in previous learning environments. The NSA is an academic intervention programme designed to support and increase opportunities for high school students to successfully complete graduation requirements and progress to future studies at two or four year colleges, universities or vocational programmes. Students attending NSA are often branded and labeled as students unable to cope with the learning demands of traditional comprehensive schools. Most have been on an academic trajectory characterized by poor achievement, poor attendance, and unacceptable behavior in and out of school. The stories of students prior to attending NSA are often filled with anger, confusion, pain, transience, poor instruction, and dysfunction.The enrollment at NSA during the 2011–2012 academic year was 460 students. Students matriculate for one year, and, after their tenth-grade year with demonstrated improved academic achievement, may return to their comprehensive high school. However, most students do not return to their comprehensive high school and instead choose to remain at NSA. NSA offers the same required academic courses that are offered in the comprehensive high schools in Northwood Public Schools. The environment at NSA embraces a small-school structure and a very strong emphasis on student–teacher relationships. The school is proud of its 85% attendance rate and 76% graduation rate. However, the students at the NSA have not met the Adequate Yearly Progress (AYP) assessment required by the federal government for the past two years. For example, in 2010–2011, the eleventh graders at NSA were 14% proficient in English Language Arts/Reading and only 1.5% proficient in mathematics as measured on the Michigan Merit Exam. Proficiency measures a students' basic level of knowledge in a given curriculum area. Scores are divided into four performance levels: Not proficient, partially proficient, proficient and advanced. Students who place in either the proficient or advanced levels are considered to be “proficient or above” in that subject (MDE, 2007). It is evident from the state assessment results that students at NSA are not demonstrating understanding of the basic concepts and skills in mathematics and English that are required for science learning and achievement.
Chemistry classroom
The classroom is not a traditional high school chemistry laboratory setting. Teachers share a common preparation and science, chemical, and equipment storage area. The classroom combines a collaborative seating arrangement that supports independent and group collaborative inquiries. The students work in collaborative groups of four to five students. A Smartboard is used to supplement the science excursions and experiences that the teacher may not be able to provide directly to her students.Participants of the study
The participants in this study consisted of 48 students (23 boys and 25 girls, 15 to 18 years of age), African–American and mostly from economically disadvantaged households. These students were enrolled in two science classes at NSA. However, due to the transient nature of students entering and leaving NSA, not all 48 students completed both the pre- and post-interventional test. Both control and Experimental groups of science students were taught a chemistry unit on acids and bases by the same teacher. Each class received equal instructional time throughout the semester unless it was a school or district early release or vacation day. To establish which class received the intervention a coin was flipped. Seventeen and 22 students in the experimental group and control group, respectively, completed both the pre- and post-interventional test. A shift in the number of students participating in the study from 48 students at the beginning of the study to the final sample of 39 students, was due to many reasons. At an alternative school students have a higher incidence of attendance concerns such as court appearances, suspensions, personal home obligations and job responsibilities that impact the daily matriculation of students at school. Therefore, statistical analysis was limited to assessment data results for students in both the control and experimental groups that completed both the pre and post intervention test.The chemistry teacher, Bonnie (pseudonym), is a veteran science teacher in the Northwood School District. As a result of district staffing changes due to budget reduction efforts, she and many other teachers were displaced from courses and grade levels they traditionally had taught. Therefore, the 2010–2011 academic year was Bonnie's first year teaching chemistry at the high-school level. Prior to this assignment, Bonnie taught biology and an integrated physical and earth science course. This transitional period was a premiere time for her to learn about the CKCM so as to enhance her expertise in teaching high-school chemistry. She mentioned that the author of the CKCM made constant reference to the notion of empathy underpinning the CKCM during a district-wide professional development session consisting of secondary science teachers in Northwood Public Schools. Recognizing the value of the instructional CKCM that embraced intellectual empathy, Bonnie was encouraged and motivated to pursue a deeper understanding of it. She wanted to implement the CKCM lesson sequence on acids and bases in the fall of 2011 and employ all of the lessons she learned during the previous academic year to reflect on her practice. We provided appropriate assistance to the teacher in the following ways: (a) the development of exploration activities, (b) the development of phenomenographic categories of description, and (c) the construction of a matrix matching categories of description with the State Science Standards and acid–base-lesson sequence using students' conceptions of acids–bases, neutralization, and students' views of the effects of acid rain on the environment.
While Bonnie was learning the CKCM to teach chemistry, she was also preparing for the National Board Take One!, which is an introduction to the National Board Certification and serves as one of the requirements for teachers interested in pursuing full National Board Certification. National Board Take One! is a professional development initiative directed by the National Board for Professional Teaching Standards and has entered into a partnership with the Northwood School District to improve teacher practice. Professional development through the National Board Take One! supports classroom-based research. Engaging teachers in the process of posing questions and reflecting on their practice is integral to the Take One! Bonnie was part of this experience during the 2010 and 2011 academic years. Because Bonnie is experienced in experimental design and conducting controlled experiments, she began her personal quest to study her practice using the CKCM in the context of the National Board Take One!
Teaching in the experimental and control classes
The school follows a block schedule rotation, which means the chemistry teacher taught each of her classes for 90 minutes twice weekly and every other week she had an additional 55 minute instructional period with the students. The implementation of the intervention occurred over seven weeks. The instruction started in late September and concluded in mid-November of 2011.The experimental and control groups of students were both taught an acid–base lesson sequence aligned to the Northwood Public Schools Curriculum Framework. The District curriculum framework was directly aligned to the State High School Curriculum (MDE, 2007) for secondary chemistry. The control group was taught an acid–base lesson sequence with the same objectives and a traditional teaching methodology that included mostly lecture, direct instruction that focused on concepts, note taking, and a high reliance on the textbook that included the teacher asking students to read, turn to selected pages and answer questions. The control class also experienced virtual lab experiences, and tests. In the control class students did not engage in collaborative discourse with each other and with the teacher, it was all very directed. Aligned to the District's content standards and objectives for chemistry, as the teaching intervention for experimental group (see Table 1), Bonnie used the CKCM acid–base lesson sequence (see Table 2).
| Phenomenographic categories of students' conceptions of acids and bases | Science benchmarks/content expectations | Essential questions | Science activities |
|---|---|---|---|
| Characteristics of acids and bases | Describe tests that can be used to distinguish an acid from a base. | What tests can be used to distinguish an acid from a base? | Perform pH experiments to identify acids, bases and neutral substance. |
| Classify various solutions as acidic or basic, given their pH. | Based on the pH values of various solutions, how would you classify acids and bases? | Demonstrate how to use a pH scale, i.e. weak and strong acids, bases, and neutral. | |
| Recognize formulas for common inorganic acids, carboxylic acids, and bases formed from families I and II. | What are the various acids, carboxylic acids, and bases formed from families I and II? | Determine if a household solution is an acid or base and rank the relative strength according to pH | |
| Identification of reactants and products in a neutralization reaction | Predict products of an acid–base neutralization. | How do acids and bases help your body maintain a state of equilibrium? | Acid–base titrations |
| Reaction of chemicals impacting the environment | Explain why lakes with limestone or calcium carbonate experience less adverse effects from acid rain than lakes with granite beds. | Why do lakes with limestone or calcium carbonate beds experience less adverse effects from acid rain than lakes with granite beds? | Perform an experiment to test whether the presence of soil in water will influence the pH of water and will change when an acid or base is added |
| Explain why sulfur oxides and nitrogen oxides contribute to acid rain. | How do sulfur oxides and nitrogen oxides contribute to acid rain? | Perform an experiment to test whether the presence of soil in water will influence the pH of water and will change when an acid or base is added | |
| Lessons based on students' conceptions | Activities | CKCM strategies |
|---|---|---|
| Phase 1: exploring and categorizing | ||
| Lesson 1 Exploration and categorization of students' conceptions of acids and bases |
After individually interviewing 10 students with four acid–base activities outside the class, the teacher explored all students' conceptions of acids and bases through writing and drawing on specially designed worksheet. | Exploration and categorization |
| Lesson 2 Students' awareness of their conceptions |
The teacher discussed the descriptive categories of acids and bases with students. | Awareness of students' conceptions of acids and bases was revealed through discussion |
| Phase 2: constructing and negotiating | ||
| Lesson 3 Determination of pH of a variety of acids and bases. |
Students were given a variety of common substances and asked to test the substances using litmus and pH paper. Students ordered substances based on their strength. This activity was the precursor to the directed instruction related to the use of indicators and pH. | Student–student, student–teacher discourse |
| Lesson 4 Determination of a pH of a variety of acids and bases with various indicators |
The teacher reviews prior lesson on indicators and testing of common substances and re-visits the pH scale. The teacher introduces pH paper, litmus paper and universal paper as different types of indicators. Students predict, observe, and explain as they engage in a guided activity testing a variety of acids and bases with different types of indicators and recording results. The teacher asks students to note patterns. The teacher allows students to explore testing other substances including substances that they have in their personal possession such as lip gloss, lotion, water etc. | “POE”, Predict, Observe and Explain conceptual change inquiry strategy |
| Flexible grouping to facilitate student peer discourse | ||
| Lesson 5 Creation of a pH scale |
Students prepared their own cabbage juice indicator and tested various pre-selected substances with the cabbage juice. Students observed color changes and collaboratively determined the strength of the acids and bases and constructed a pH scale. | Inquiry and student discourse |
| Lesson 6 Determination of pH of unknown substances |
Students designed and implemented their own experiment using seven unknown substances. | Inquiry and student discourse |
| Lesson 7 Conceptual understanding of Hydrogen and hydronium ions |
Students were engaged in a series of learning activities that addressed the function of potential hydronium (pH). The teacher later engaged students in a discussion about ions and led them to an understanding that acids generate hydronium ions in aqueous solutions and bases generate hydroxide ions in aqueous solution. | Explanation of theoretical ideas of acids and bases |
| Lesson 8 Conceptual understanding of neutralization |
Students observe teacher demonstration of an acid combining with a base. The teacher engages students in constructing an equation to represent the neutralization reaction, highlighting the hydrogen ion and hydronium ion. | Teacher demonstration and large group interpretive discussion |
| Lesson 9 Conceptual understanding of neutralization |
Students conducted a neutralization activity by combining a common acid (HCl) with a base (NaOH) to form salt and water. They begin to connect the idea of dissociation in lesson 8 to neutralization. | Student inquiry, small group peer discourse |
| Teacher explanation and probe for deeper understanding | ||
| Lesson 10 Titration lab |
Students conducted a titration lab to reinforce their emerging understandings about neutralization and concentration of acids and bases. | Student inquiry, small group peer discourse |
| Teacher explanation and probe for deeper understanding | ||
| Phase 3: extending and translating | ||
| Lesson 11 Exploration of students' conceptions of acid rain |
Students were led in an engaging conversation about acid rain. Students collected water and soil samples from around the school. Students tested the soil samples for pH and charted their results. |
Teacher–student discourse – predict, observe, explain (POE). Student inquiry and small group discourse |
| Phase 4: reflecting and assessing | ||
| Lesson 12 Students' depictions of acids and bases |
As a formative assessment, the students created children's books to teach about acids and bases. | Students' drawings for conceptual understandings |
| Teacher probe for deep understanding | ||
| Lesson 13 Exploration of students' post-intervention conceptions of acids and bases |
Teacher explored students' post-intervention conception of acids and bases through the same worksheet used in prior-intervention. | Post-teaching conceptions |
| Teacher explored the same 10 students' conceptions of acids and bases through individual interviews after the intervention. | ||
| Lesson 14 Final test |
Teacher assesses student achievement in a unit on acids and bases ABA-T | Post-test |
Research design
An exploratory sequential mixed-methods design (Creswell, 2003), including both qualitative and quantitative inquiry methods, was employed in this study. To determine students' conceptual changes, “phenomenographic individual interviews” (Ebenezer et al., 2010) were conducted prior- and post-intervention. To measure achievement, a quasi-experimental pre- and post-test design (Campbell and Stanley, 1963) was used. The study used “retrospective data analysis” (Shavelson et al., 2003) of the students' conception and achievement data that the teacher had gathered for the National Board for Professional Teaching Standards Take One!Data collection
Data collection is represented in Table 3.| Data collection by the teacher | Retrospective data analysis by the researchers | ||
|---|---|---|---|
| Qualitative data | Quantitative data | Qualitative data | Quantitative data |
| Audiotape prior- and post-interventional interviews. | Administer pre- and post-intervention achievement test to both control and experimental groups. | Transcribe and analyze prior- and post-interventional interviews. | Analyze the pre- and post-intervention achievement test data from both control and experimental groups. |
To explore urban African–American alternative students' conceptions of acids and bases, to determine tasks and questions for the in-classroom specially-designed worksheet to explore all students' conceptions, and to determine conceptual changes, Bonnie used a qualitative (phenomenographic) assessment tool consisting of three tasks (pointing to a lemon, liquid soap, and the reaction between lemon juice and baking soda) and related questions that focused on the concepts of acids, bases, and neutralization, respectively. These topics were selected for the prior- and post-interventional interviews because they constituted the major themes of the state's science curriculum (MDE, 2007; also see Table 1).
Bonnie randomly selected 10 students from the experimental class and explored their conceptions using the above three tasks. To identify students for this random interview, all students in the experimental class were assigned a number and were then randomly selected by number to participate in the interview. This was done prior to the implementation of the instructional intervention. Each student's interview was audio-recorded. Based on the data gathered from these prior-interventional interviews, Bonnie constructed a worksheet with similar activities and questions and administered it to all the students in the class to explore their conceptions. Because the alternative students' written responses were sparse, only the interview data have been used to report this study.
The Acid–Base Achievement Test (ABA-T) (see Appendix), aligned to concepts in the Northwood Common Chemistry Assessment, was designed by the teacher. The ABA-T was administered to both the control and experimental groups during the same week at the beginning and end of the lesson sequence.
Conceptual change analysis
First, all 10 prior- and post-interventional individual interviews were transcribed verbatim. The transcripts from the prior-intervention interviews were reviewed several times. Passages with similar responses within each interview were colour coded. Each group of the colour-coded passages was assigned a special label to depict the overall meaning. The descriptive categories were identified based on students' conceptions of acids and bases after several rounds of comparisons of categories for internal consistency and consistency with the interview data as admonished by Ebenezer et al. (2010), Orgill (2002), and Sjostrom (2002). The same rigorous procedure was conducted with the post-interventional individual interview data. Additional descriptive categories were developed from the post-interventional interview transcripts. Frequencies were tallied for both prior- and post-interventional data.Achievement data analysis
The study compared the results from the quantitative analysis of pre- and post-interventional tests on acid–base concepts for a sample group of 39 students, including an experimental group (n = 17) and control group (n = 22). The first analysis compared the pre-intervention test scores between the two groups to determine the statistical equivalence of the groups prior to beginning the intervention. This analysis was needed because the students could not be randomly assigned to the experimental and control groups due to classroom assignment. The post-interventional test scores were compared between groups using t-tests for independent samples. This analysis was considered appropriate, as the groups were statistically equivalent prior to starting the intervention. The change in the experimental groups' scores from pre- to post-intervention test was examined using t-tests for dependent samples. This change was used to determine the effects of the intervention on the knowledge that students gain from participating in the intervention. The control group was tested at the same time as the experimental group, but their change scores were not compared, as this was not a focus of the study. However, the post test scores for the control and experimental groups were compared to determine the extent to which the CKCM improved the experimental group's conceptual understanding of acids and bases.Results and discussion
The purpose of this study was to investigate the effects of the intellectually caring model (CKCK) intervention on African American alternative high school students' conceptual change and achievement following the enactment of a lesson sequence on acids and bases. The results of students' prior- and post-interventional data provide evidence on conceptual change and achievement. Tables 4 and 5 present the categories of descriptions and the frequency of students' conceptions.| Descriptive categories | Prior | Post | F | |
|---|---|---|---|---|
| Examples of students' expressions | Examples of students' expressions | Pre | Post | |
| Students' conceptions of acids | ||||
| Sour taste | Taste sour because of juice | Sour taste, its highly acidic | 15 | 5 |
| Has a twang like skittles | ||||
| Irritates and tingles | If juice squirts in eye it will burn | 11 | 0 | |
| Makes the taste buds tingle | ||||
| Acid turns paper red | Acid turns paper red | 0 | 12 | |
| pH value for acid (below 7) | Acids have a pH that is below seven | 0 | 8 | |
| A pH scale can determine whether something is an acid or a base | ||||
| I remember we used cabbage to make a pH scale to determine if it was an acid or base | ||||
| Students' conceptions of bases | ||||
| Soapy taste | I taste soap | Bitter | 9 | 2 |
| Slippery to touch | It is slippery | Smooth and slippery | 16 | 2 |
| Characteristics of base | It burns | 0 | 2 | |
| pH value for base (above 7) | Bases have a range from eight to fourteen | 0 | 9 | |
| Students' conceptions of neutralization | ||||
|---|---|---|---|---|
| Descriptive categories | Prior | Post | F | |
| Examples of students' expressions | Examples of students' expressions | Pre | Post | |
| Reactants | Baking soda and lemon juice | 4 | 0 | |
| Reaction of chemicals | See reaction of chemicals when the lemon juice hits the baking soda | Product sodium chloride | 1 | 3 |
| Neutralization of acid and base | When you combine an acid and base it neutralizes and yields salt and water | 0 | 5 | |
| Dissociation of acid and base | Dissociation occurs when acids and base break apart in water | 0 | 7 | |
Based on the descriptive categories presented in Tables 4 and 5 above, the study observed the following trends: (a) a change in the number of categories of description, (b) a shift in language use from everyday talk to chemical talk and (c) a hierarchy of chemical knowledge. Each knowledge claim is characterized with pertinent examples taken from prior- and post-intervention interview excerpts.
Knowledge claim one: a change in the categories of description
Lemon tastes “sour” is the common expression made by all students in the prior-intervention. When asked why lemon is sour, 15 students were not sure what was in the juice but confident that the juice had something in it, or something added to it, or likened it to the taste of “Skittles.” that made it sour. Twelve students stated that acids are sour and that is why they “tingle,” “irritate,” or “burn.” The reason for acids being sour because of something added to it during the post-interventional interviews occurred only five times. In the post-intervention, the sour taste was attributed to the “acidic” nature of the lemon. The property of acid that it “burns” remained in the minds of nine students. Prior-and post-intervention Excerpts 1 and 2 characterize students' understanding.Excerpt 1 – Shelly (prior-interventional interview)
1. T: Alright. You mentioned the lemon has a sour taste. Why is it that you think it has a sour taste?
2. S: I'm not sure, but it tastes sour and probably because it's a citrus fruit. That's probably why it's sour. I'm not sure exactly why a lemon is sour. You can use lemons in cooking, such as shrimp scampi or different pasta dishes, to give it a little twang.
3. T: The twang. Now let's talk a little bit about that twang. What is it?
4. S: It's just, just a sour taste or twang. That's why you make lemonade with it.
Shelly talks about the “sour” taste of lemon because of the “twang” nature of the “citrus fruit” (2). She supports her argument with her experience in cooking dishes with lemon to give that “twang” taste. When the teacher asks her about the twang, Shelly explains, “You make lemonade because of its “sour taste or twang.” Now observe what Shelly states after studying about acids and bases (see Excerpt 2):
Excerpt 2 – Shelly (post-interventional interview)
1. T: Okay, what do you know about lemon juice?
2. S: It has a sour taste, it's highly acidic.
3. T: You said it's acidic. What can you tell me about acids?
4. S: Um…Acids are typically sour… . Using litmus paper, acids turn red.
Shelly does not use the term “twang.” She retains the sour-tasting aspect of acids. She states that lemon is “highly acidic”. She substantiates her understanding of what an acid is by pointing out a classic test for acids, which is acids turning litmus paper red. It is evident that Table 5 indicates that students in their post-interventional interview talked about the litmus paper turning red (see Shelly, 4; and Justin, 2), testing the acid with a pH scale (Justin, 4,5), and neutrality of a substance if the test indicates 7 (Justin, 6). These assertions are evident in Excerpt 3.
Excerpt 3 – Justin (post-interventional interview)
1. T: What do you think about acids?
2. S: If you test it. If it is an acid, it will turn red.
3. T. Okay, so how do you test it?
4. S: A pH scale.
5. T: Oh! Well, talk to me about the pH scale.
6. S. Um, okay. A pH scale can determine whether something is an acid or a base. I know the middle number is seven and that means it's like neutral.
Justin describes his understanding about testing substances to determine if they are acids. He says “it will turn red” (2), as he reflects on the use of litmus paper and implies its use and to indicate his beginning understanding to pH (6).
Knowledge claim two: a shift in language use
Shift in language is illustrated with descriptive categories of students' conceptions of bases. The teacher showed dishwashing soap to explore student ideas about bases. According to Table 5, students' use of everyday language based on their sense of taste (bitter) and touch (slippery) declined from 9 to 2 and 16 to 2, respectively. Chemical characterization about soap has risen from 0 to 9. Two excerpts from the interview transcripts of Jennifer are represented to illustrate the shift in language from everyday talk to more chemical talk.Excerpt 4 – Jennifer (prior-interventional interview)
1. T: If you had to taste dishwashing liquid, what would it taste like?
2. S: Disgusting, bitter.
3. T: Okay, you mentioned that it tastes bitter. Why does the soap taste bitter?
4. S: Probably chemicals. I'm not sure which chemicals, but probably chemicals that they put in there to help get it cleaner. That's why it will make it taste bitter.
The excerpt reveals that the teacher is attempting to find out the characteristic taste of a base (1). To this Jennifer states that soap is bitter (2). She knows the taste of soap, perhaps because of tasting it, not that she classifies it as a base. She thinks that there are certain chemicals (4) that are put in it for cleaning purposes to make soap bitter (4). What does Jennifer state in her post-intervention interview?
Excerpt 5 – Jennifer (post-interventional interview)
1. T: Now, take a look at the dish of washing liquid. How would you classify it?
2. S: The dish liquid?
3. T: Right, would you classify…how would you classify?
4. S: Um, base.
5. T: Okay, tell me about a base.
6. S: Um, bitter taste.
7. T: Uh huh.
8. S: When you test, it turns litmus paper blue, has a pH of seven and greater negative charge.
9. T: Okay, and can you recall what makes up a base?
10. S: Yeah, hydroxide.
11. T: Hydroxide, and what does that look like? You mentioned that the H for an acid.
12. S: OH negative
Jennifer's post-interventional interview responses show signs of increased understanding of bases because she uses chemical language to describe bases. The teacher asks Jennifer to classify liquid soap. Jennifer without hesitation states that it is a base (4). When the teacher asks Jennifer to tell more about the base, Jennifer said that it: has a bitter taste (6), turns litmus paper blue (8), has a pH of 7 and greater (8), has a “negative charge” (8), is a “hydroxide” (10), and is OH negative (12). Jennifer was able to state at least six properties of a base when liquid soap was shown to her at the post-intervention interview. This sort of chemical talk by an alternative high school student is impressive.
Knowledge claim three: a hierarchy of knowledge
Students explored their ideas related to combining an acid with a base. There were four descriptive categories identified based on students' conceptions of neutralization from prior- to post-interventional interview. These categories are: (a) reactants (b) reaction of chemicals (c) neutralization of acid and base and (d) dissociation of acid and base (see Table 5). These categories of description depict a hierarchy of knowledge. Excerpt 6 illustrates the student's focus on the reactants.Excerpt 6 – Stephanie (prior-interventional interview)
1. T: Yeah, with the baking soda and the lemon juice, what happens?
2. S: Because that's sour and that's bitter.
3. T: Yeah, so what's happening? Why do you think it fizzed?
4. S: Because of the baking soda.
5. T: What's happening? Can you explain that?
6. S: Uh-uh. No, because they're both different. I think the lemon juice.
The reaction is obvious because fizzing is visible. However, Stephanie is focusing only on the reactants and not the products. Despite the teacher's simultaneous talk about baking soda and lemon juice, the student talks about baking soda (4) then Stephanie focuses on the lemon juice (6).
In contrast, Gary describes what he saw when the lemon juice and baking soda were combined and begins to state what happens and why. See Excerpt 7 for this evidence:
Excerpt 7 – Gary (prior-interventional interview)
1. S: A different analysis. A totally different reaction of chemicals in it because I see the reaction when the lemon juice had hit the baking soda, came like a different reaction with more bubbles. I think that they are mixed together and it's a totally different ingredient now.
2. T: Okay. Do you know what those bubbles are?
3. S: Those are chemicals from the lemon juice.
It is evident from Gary's responses: that he has rudimentary knowledge about chemical reaction. He explains “there is a totally different ingredient now” (1) “when the lemon juice had hit the baking soda” (1).
In the post-interventional interviews, there were examples of student expressions that showed increased understanding supporting the fact that there was development of knowledge: simple to sophisticated. Shelly offers a simple explanation when she talks about acids and bases (see Excerpt 8).
Excerpt 8 – Shelly (post-interventional interview)
1. T: In terms of acids or bases…very reactive with them.
2. S: Sodium Chloride
3. T: Okay, what about sodium chloride? …That brings me to what happens when you combine an acid and a base?
4. S: They become neutral.
Shelly begins to share her emerging understanding related to the process of combining an acid and base. She states that they become neutral (4). In contrast, Rita's explanation is more sophisticated. She describes her understanding of the neutralization process and products (see Excerpt 9).
Excerpt 9 – Rita (post-interventional interview)
1. T: When you think of substances combining…
2. S: It's when, I guess they combine, and they make salt water.
3. T: Okay, when what combines?
4. S: Uh, like hydrochloric acid and hydroxide
5. T: Ok, is it specifically hydrochloric acid and sodium hydroxide, or is there a concept behind that? Is it only hydrochloric acid and sodium hydroxide that will produce a salt and water?
6. T: Let's take a look at…
7. S: Oh, phenolphthalein. I remember that.
8. T: What is that?
9. S: It's the…isn't it a combination of the two…these two items?
10. T: Just tell me, when you saw the phenolphthalein how was it used?
11. S: Uh, for neutralization.
12. T: For neutralization?
13. S: Uh huh.
14. T: Well, what's neutralization?
15. S: It's like…when both cancels out. Like a chemical cancels out another chemical to make like a neutralize…it's like neither a base or acid.
Rita, instantly states when hydrochloric acid and hydroxide combine (4), they make salt water (2). She remembers that phenolphthalein has something to do with this (7). But she does not refer to it as an indicator. When the teacher asks how it is used (10), then she talks about the neutralization (11). This learning may result from the titration lab that students participate in. Rita explains neutralization as follows: “It's like…when both cancels out. Like a chemical cancels out another chemical to make like a neutralize…it's like either a base or acid” (15).
Talia takes us further with respect to the hierarchy of knowledge development. Talia, describes the process as follows.
Excerpt 10 – Talia (post-interventional interview)
1. T: Anything that you found interesting or any comments about this unit on acids and bases?
2. S: Dissociation.
3. T: Dissociation! I'm glad you brought it up. Talk to me about dissociation.
4. S: Um, okay. Say…like…okay, you've got water, you got acid, and then a base and they break apart.
5. T: Yes.
6. S: And.
7. T: What's being separated? Specifically in terms of particles?
8. S: Hydrogen ions.
9. T: Ye, and what type of ion charge is associated with an acid?
10. S: Positive ions…and with a base is hydroxide…negative hydroxide.
11. T: Can you recall the names of those ions…the name of the positive ions?
12. S: Um, cations.
13. T: And, the negative.
14. S: Anions
15. T: Okay. What about the elements for an acid...that represents an acid and the elements that represent a base?
16. S: Hydrogen, the H+, and OH−
The teacher asks a very general question about the unit on acids and bases? Immediately Talia references the term “dissociation (2). The teacher sounds surprised and takes the opportunity to probe Talia about what she means by dissociation (3). Talia begins by saying that acid and base break apart (4). Upon the teacher's further probing, Talia names the ion (Hydrogen ions), when dissociation occurs (8). The teacher talks about the ionic charge associated with an acid. Student talks about the positive ion of an acid and negative ion of hydroxide (10). The teacher and Talia talk about anion and cation (12–14). The teacher wants to make sure about the elements of an acid and a base (15). Talia represents the charge for the hydrogen ion and the hydroxide ion (16).
Student achievement
To determine whether students in the experimental and control groups were similar prior to starting the intervention, a pretest was completed that measured students' conceptions of “acids and bases.” The scores on the prior test were obtained and compared using t-tests for two independent samples. The results of this analysis are presented in Table 6.| Group | N | Mean | SD | DF | t-Value | Sig |
|---|---|---|---|---|---|---|
| Control | 22 | 5.18 | 2.26 | 37 | 1.13 | 0.267 |
| Experimental | 17 | 4.41 | 1.91 |
The results of the t-test for two independent samples provided no evidence of statistically significant differences between the control and experimental groups on the prior-teaching test Acids and Bases Achievement Test, t (37) = 1.13, p = 0.267. This result indicated that although the control group (m = 5.18, sd = 2.26) had higher scores than the experimental group (m = 4.41, sd = 1.91), the difference was not substantial enough to be statistically significant. Based on this finding, the two groups were considered statistically equivalent prior to starting the intervention.
Following completion of the intervention, the same test was administered to the two groups, control and experimental. The scores on the post-teaching test were compared between the two groups using a t-test for two independent samples. Table 7 provides results of this analysis.
| Group | N | Mean | SD | DF | t-Value | Sig |
|---|---|---|---|---|---|---|
| Control | 22 | 7.50 | 1.97 | 37 | 3.16 | 0.003 |
| Experimental | 17 | 10.65 | 4.11 |
The results of the t-test for independent samples comparing the experimental group (m = 10.65, sd = 4.11) and the control group (m = 7.50, sd = 1.97) on post-intervention test Acids and Bases Achievement Test were statistically significant, t (37) = 3.16, sd = 0.003. This result indicated that, following the intervention the mean scores for the experimental group were significantly higher than the mean scores for the control group.
To determine the extent to which the experimental group's understanding of acids and bases changed from pretest to posttest, the mean scores for the pretest and posttest were compared using t-tests for dependent samples. Table 8 presents the results of this analysis.
| Time | N | Mean | SD | DF | t-Value | Sig |
|---|---|---|---|---|---|---|
| Pretest | 17 | 4.41 | 1.91 | 16 | 7.91 | <0.001 |
| Posttest | 17 | 10.65 | 4.11 |
Implications
The purpose of the study was to determine the effect of the CKCM (Ebenezer et al., 2010) on the alternative high school students' conceptual changes and achievement in a unit on acids and bases. Students' conceptions of acids and bases prior to the teaching intervention were compared to the conceptions after the enactment of a caring relational conceptual change anchored in phenomenography. Similarly, the experimental and control students' achievement results based on district-wide common assessment in a unit on acids and bases were compared in two ways. The pre- and post-intervention test results were compared within the experimental group and with the control group. Based on the results of the use of the CKCM with alternative high school students, implications are drawn advocating the adoption of an intellectually caring conceptual change CKCM for (a) reaching the often unreached mind, (b) developing simple chemical phrases into coherent chemical explanations, and (c) achieving alternative students' success in traditional test.Reaching the often unreached mind
The phrase “alternative high school students” invokes hopeless feelings about such students that they can be seldom reached intellectually. This is because alternative students enter high school without experiencing academic success. We launched this study believing that alternative students are very capable of learning science and deserve the support of teachers who will place instructional value on their ideas. The teacher's wisdom to use an intellectually caring conceptual change CKCM with students who have not been reached through traditional educational environments during her preparation for the National Board Take One! is a step in the right direction.Evidence in this study suggests that using the intellectually caring conceptual change model – the CKCM (Ebenezer et al., 2010), theoretically rooted in the variation theory of learning that conjectures learning is relational and involves a qualitatively different approach to understanding a phenomenon (Marton and Booth, 1997), is effective in facilitating African–American alternative high school students' conceptual changes and achievement.
Much care was taken to explore students' conceptions before and after the acid–base lesson sequence. Students' prior-conceptions of acids and bases and neutralization were systematically linked to the district and state science curriculum standards (see Table 1) so that these alternative students have equal opportunity to learn chemistry. Furthermore, the teacher tracked students' conceptual change by looking at students' prior- and post-interventional conceptions.
Conceptual change reflected a change in descriptive categories, a shift in language use from simple to more sophisticated, and a hierarchy of knowledge (see Tables 4 and 5). The most apparent conceptual change occurred in the descriptive category of “neutralization of acid and base” (Excerpt 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and “dissociation of acid and base” (Excerpt 10
5) and “dissociation of acid and base” (Excerpt 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 8 and 10). Students demonstrated increased clarity in their thinking and responses to questions related to acids and bases. The data support the absence of these conceptual ideas prior to the intervention; however, after the intervention, the addition of new conceptions confirms conceptual change according to phenomenographic notion of conceptual change. These examples of change infer the nature of conceptual change that the NSA alternative students experienced when learning a lesson sequence on acids and bases.
4, 8 and 10). Students demonstrated increased clarity in their thinking and responses to questions related to acids and bases. The data support the absence of these conceptual ideas prior to the intervention; however, after the intervention, the addition of new conceptions confirms conceptual change according to phenomenographic notion of conceptual change. These examples of change infer the nature of conceptual change that the NSA alternative students experienced when learning a lesson sequence on acids and bases.
This study developed a phenomenography of acids and bases using the students' prior-conceptions like the Ebenezer and Erickson's (1996) study on solution chemistry. It also tracked conceptual change following Ebenezer and Erickson (1996) on solution chemistry and Ebenezer et al.'s (2010) study on the concept of excretion. Adopting a caring conceptual change model, although rare, has been highly successful as exemplified by Ebenezer's former studies, and this study with the CKCM is no different although used with alternative high school students. Exploring the value of a caring model in supporting teachers who also believe academic success is possible with all learners was the impetus for testing the CKCM with this group of alternative high school students. What is gleaned from this study is that facilitating a caring conceptual change model is important for students similar to those who attend the NSA.
Developing simple chemical phrases into coherent chemical explanation
An example of a simple chemical phrase was when students denoted the dissociation process of acids and bases-giving birth to new chemical species, the H+ and OH−. The alternative students have begun to articulate chemical language, such as ions. It is evident that the teacher also appreciated the students' use of chemical language (see Excerpt 10). A shift from everyday use (e.g., twang) to simple chemical talk (e.g., ions) is noteworthy and commendable. However, we cannot remain complacent about the alternative students' attainment to express their chemical understandings in simple chemical phrases. Teachers need to strive for developing students' simple chemical phrases such as H+ and OH− into coherent chemical explanation of neutralization in ionic terms.Chemistry educators have long realized that students struggle to differentiate between macroscopic observation and sub-microscopic explanation and the need to help students to move seamlessly among the three types of chemical knowledge – the macroscopic, the sub-microscopic, and the symbolic (Driver et al., 1994; Duit and Treagust, 2003; Kind, 2004). This study has provided a platform to discuss the importance of not only differentiating among these types of knowledge and mitigating the difficulties that students have in the usage of this knowledge, but also pointing to the greater aim of helping students to develop and articulate coherent chemical explanations. There was change in the sophistication of the expressed ideas. Instead of saying, “when the lemon juice hits the baking soda” (Excerpt 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), students following the intervention began to use more sophisticated and appropriate scientific language, such as “when you combine an acid and base, it neutralizes and yields salt and water” (Excerpt 9
1), students following the intervention began to use more sophisticated and appropriate scientific language, such as “when you combine an acid and base, it neutralizes and yields salt and water” (Excerpt 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Replacing common language with more sophisticated language directly aligns with the need to explore ideas and expressions of the students prior to teaching the scientific language. Brown and Ryoo (2008) have suggested that when we explore and consider students' social language that they commonly use, it is the first step in enhancing their understanding of new concepts and express these in specialized or academic language. Our concern is that not only is the development of specialized language important, but developing specialized language into coherent explanation is even more important (Thagard, 1992). It is only then that we can achieve a nobler vision of making the evidence-explanation connection leading to a chemical argument (Duschl and Grandy, 2008).
2). Replacing common language with more sophisticated language directly aligns with the need to explore ideas and expressions of the students prior to teaching the scientific language. Brown and Ryoo (2008) have suggested that when we explore and consider students' social language that they commonly use, it is the first step in enhancing their understanding of new concepts and express these in specialized or academic language. Our concern is that not only is the development of specialized language important, but developing specialized language into coherent explanation is even more important (Thagard, 1992). It is only then that we can achieve a nobler vision of making the evidence-explanation connection leading to a chemical argument (Duschl and Grandy, 2008).
For developing students' simple chemical talk into coherent explanation to begin, teachers need to listen very carefully and empathetically to students' use of simple chemical phrases to convey their chemical understanding. Just because students use one or two words to express their understanding, a beginning chemical articulation should not be treated trivially. Gradually developing chemical language from simple phrases to more complex forms of expressions should be a priority in a chemistry classroom. Any wonder, the Common Core Assessment for Language Literacy is promoting the idea that writing and reading should be taught in every subject matter, which is a welcome idea. For example, Lindo (2006) asserts that literacy must be emphasized in subject areas. This should be done in a manner that does not look down on the student, but rather celebrates successes through small steps in language use. To achieve this, teachers should listen carefully to student talk and strategically help students to reformulate their thoughts, both oral and written, to convey the meaning they are attempting to convey. Many alternative students demonstrated their chemical understanding, but their articulation does not convey the sophisticated level (coherent chemical explanation) that is desired in secondary chemistry class.
Not only do teachers listen carefully to students' talk, but students need to realize that their ideas and the language they use to express their ideas will not be dismissed or looked down. To dismiss and devalue the language or experiences students share is not complementary to the caring CKCM. Ignoring everyday and/or simple chemical talk not only shuts down the desire of students to share their understandings but also denies a forum for the teacher to begin to help students develop their language into coherent chemical explanation through social discourse in the classroom. Creating an intellectual community for social discourse supports chemistry learning. A teacher needs to create a learning environment where respect and care are expected, demonstrated, and perceived not only by the teacher but also equally by students. It is important for teachers to have an awareness of the impact of classroom talk and to facilitate the development of conceptual ideas and usage of more sophisticated conversation in the science classroom (Morton, 2012).
Achieving alternative students' success in traditional test
The teacher implemented the intellectually caring conceptual change CKCM with the experimental group and implemented traditional classroom pedagogy with the control group. The results of the t-test for independent samples comparing the experimental group (m = 10.65, sd = 4.11) and the control group (m = 7.50, sd = 1.97) on post-intervention test Acids and Bases Achievement Test was statistically significant, t (37) = 3.16, p = 0.003. Following the intervention of the CKCM, the mean score for the experimental group was significantly higher than the mean score for the control group. The ABA-T consisted of conceptual questions. This study was conducted in one high school with a small number of students. Significant changes and improvements in the students' conceptual change and achievement in a chemistry unit on acids and bases in the short-term may have reflected a Hawthorne effect. This might be because of the increased intellectual and emotional care students received to work harder to master the chemical concepts. Before the potential of the CKCM can be confidently evaluated, there needs to be further examples of studies conducted in diverse alternative students' learning environments supporting the approach. Although the study considered only African–American students the findings seem likely to be relevant to disenfranchised groups throughout the educational system.Schroeder et al. (2007) conducted a meta-analysis of national research on the effects of teaching strategies on student's science achievement. In the list of 10 strategies, not even one strategy pertains to conceptual change teaching and learning that impacts student achievement. As indicated earlier in the need for this study, conceptual change studies are beginning to have a positive impact on student science achievement (Sungur et al., 2001; Eryilmaz, 2002; Demircioglu et al., 2005; Ebenezer et al., 2010). This is the second time a caring conceptual change model has been tested for achievement. It is a bold move to subject the CKCM to study its effect on alternative high school chemistry learners. The results of this study encourage us to recommend that similar studies should be conducted to test the efficacy of the CKCM to improve student achievement, including the alternative high school students.
Appendix – Intellectual caring model
Appendix A: Acid–Base Achievement Test (ABA-T)| Directions: Do not write on this test. Record your answers on a scantron. | |||||
| 1. | A group of scientists stumbles upon a lake found to have a basin mostly consisting of limestone (CaCO3) in an area of the United States known for its problems with acid rain. The lake should have a pH of ___. | ||||
| a. 6–8 | b. 2–4 | c. 9–11 | d. None of these | ||
| 2. | Hydroxides of Group 1 metals ___. | ||||
| a. are all strong bases | c. are all weak bases | ||||
| b. are all acids | d. might be either strong or weak bases | ||||
| 3. | Strong bases produce ___. | ||||
| a. small quantities of H+ ions | c. large quantities of H+ ions | ||||
| b. small quantities of OH− ions | d. large quantities of OH− ions | ||||
| 4. | The reaction HCl + KOH → KCl + H2O is a ___. | ||||
| a. synthesis reaction | c. neutralization reaction | ||||
| b. ionization reaction | d. decomposition reaction | ||||
| 5. | What is the pH of a neutral solution at 25 °C? | ||||
| a. 0 | b. 1 | c. 7 | d. 14 | ||
| 6. | A solution whose pH is 4 ___. | ||||
| a. is always neutral | c. is always acidic | ||||
| b. is always basic | d. might be neutral, basic or acidic | ||||
| 7. | A solution whose pH is 10 ___. | ||||
| a. is always neutral | c. is always acidic | ||||
| b. is always basic | d. might be neutral, basic or acidic | ||||
| 8. | The pH of a solution is 9. What is its H+ ion concentration? | ||||
| a. 1 × 10−9 M | c. 1 × 10−5 M | ||||
| b. 1 × 107 M | d. 9 M | ||||
| 9. | The pH of a solution is 10. What is its OH− concentration? | ||||
| a. 1 × 10−10 M | c. 1 × 10−4 M | ||||
| b. 1 × 10−7 M | d. 10 M | ||||
| 10. | Which substance doesn't conduct electricity? | ||||
| a. C6H12O6 | b. HCl | c. H2SO4 | d. Vinegar | ||
| 11. | Which acid is not strong? | ||||
| a. HCl | b. HCN | c. HNO3 | d. H2SO4 | ||
| 12. | Which one of the following is weak base? | ||||
| a. KOH | b. NaOH | c. NH3 | d. CH3COOH | ||
| 13. | Information for three solutions are: | ||||
 |
|||||
| Which one(s) of the above is acidic solution? | |||||
| a. #1 and #2 | b. #1 and #3 | c. #3 | d. #2 | e. #2 and #3 | |
| 14. | When performing an acid–base titration, which procedure would NOT introduce an error into the experimental results? | ||||
| a. Adding an unmeasured amount of water to the carefully measured acid sample which is being titrated. | |||||
| b. Not rinsing the burettes with the appropriate reactants after cleaning and rinsing with water. | |||||
| c. Not removing the bubbles of air from the tips of the burettes before beginning the titration. | |||||
| d. Using an indicator that changes color at a pH considerably removed from the pH at the equivalence point of the titration. | |||||
| 15. | Which one of the following is not a property of acid solutions? | ||||
| a. Solution tastes sour. | |||||
| b. Solution is a good conductor of electricity. | |||||
| c. The [H+] can be 10−2 M in solution. | |||||
| d. React with Mg to produce H2 gas. | |||||
| e. Solutions turn litmus blue. | |||||
| 16. | Determine the pH range of the salt produced in the following reaction: | ||||
| 2NaOH + H2SO4 → Na2SO4 + 2H2O | |||||
| a. 1 | b. 2–6 | c. 7 | d. 8–13 | e. 14 | |
| 17. | You are working for the Environmental Protection Agency (EPA). Which supply in your stockroom would you use to neutralize acid from a car battery? | ||||
| a. NaOH | b. HNO3 | c. HC2H3O | d. Al(OH)3 | ||
| 18. | The unit of measure used to express the concentration of an acid or base is ___. | ||||
| a. mL | b. M | c. mole | d. g | ||
| 19. | Which piece of lab equipment would give you the most accurate pH of a substance? | ||||
| a. Litmus paper | b. pH paper | c. pH probe | d. Phenophathalein | e. Universal indicator | |
| 20. | Which statement best describes your understanding of acid–base chemistry? | ||||
| a. I've seen some of this information before. | |||||
| b. I've seen a lot of this information; I just forgot how to do it. | |||||
| c. Most of the questions I've never seen before. | |||||
References
- Acar B. and Tarhan L., (2007), Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry, Int. J. Sci. Math. Educ., 5(2), 349–373.
- Becker B. and Luthar S., (2002), Privileged but pressured? A study of affluent youth, Child Dev., 73(5), 1593–1610.
- Biernacka B. and Ebenezer J., (2007), Developing grade 5 students' literacy in science: a teacher–researcher collaboration. Paper presented at the 2007 National Association of Research in Science Teaching (NARST), New Orleans, LA.
- Boo H. and Watson J. R., (2001), Progression in high school students' conceptualizations about chemical reactions in solution, Sci. Educ., 85(5), 568–585.
- Brown B. A. and Ryoo K., (2008), Teaching science as a language: a “content first” approach to science teaching, J. Res. Sci. Teach., 45(5), 529–553.
- Campbell D. T. and Stanley J. C., (1963), Experimental and quasi-experimental designs for research on teaching, in Gage N. L. (ed.), Handbook of Research on Teaching, Chicago: Rand McNally, pp. 171–246.
- Chi M. T. H. and Roscoe R. D., (2002), The processes and challenges of conceptual change, in Limon M. and Mason L. (ed.), Reconsidering conceptual change: issues in theory and practice, The Netherlands: Kluwer Academic Publishers.
- Christenson S. L., Sinclair M. F., Lehr C. A. and Godber Y., (2001), Promoting successful school completion: critical conceptual and methodological guidelines, Sch. Psychol. Quart., 16(4), 468–484.
- Creswell J. W., (2003), Research design: quantitative, qualitative, and mixed methods approaches, 2nd edn, Thousand Oaks, CA: Sage.
- Darling-Hammond L., (2000), Teacher quality and student achievement: a review of state policy evidence. Education Policy Analysis Archives 8. Center for the Study of Teaching and Policy, Seattle, WA: University of Washington.
- Demircioglu G., Ayas A. and Demircioglu H., (2005), Conceptual change achieved through a new teaching program on acids and bases, Chem. Educ. Res. Pract., 6(1), 36–51.
- Driver R., Squires A., Rushworth P. and Wood-Robinson V., (1994), Making sense of secondary science: research into children's ideas, New York: Routledge.
- Duit R. and Treagust D. P., (2003), Conceptual change: a powerful framework for improving science teaching and learning, Int. J. Sci. Educ., 25(6), 671–688.
- Duschl R. and Grandy R., (2008). Teaching scientific inquiry: recommendations for research and implementation, Rottendam, Netherlands: Sense Publishers.
- Ebenezer J., Chacko S., Kaya O., Koya K. and Ebenezer D. L., (2010), The Effects of common knowledge construction model sequence of lessons on science achievement and relational conceptual change, J. Res. Sci. Teach., 48(1), 25–46.
- Ebenezer J. V. and Conner S., (1998), Learning to Teach Science: A Model for the 21 Century, Upper Saddle River, NJ: Simon and Schuster.
- Ebenezer J. V. and Erickson G., (1996), Chemistry students' conceptions of solubility, Science Education, 80(2), 181–201. (This article was requested by The Archives of Jean Piaget, University of Geneva).
- Ebenezer J. V. and Gaskell P. J., (1995), Relational conceptual change in solution chemistry, Sci. Educ., 79(1), 1–17.
- Ebenezer J. V. and Haggerty S., (1999), Becoming Secondary School Science Teachers: Preservice Teachers as Researchers, Upper Saddle River, New Jersey: Prentice-Hall, Inc., Simon and Schuster/A Viacom Company.
- Ebenezer J. V. and Puvirajah A., (2005), WebCT dialogues on particle theory of matter: presumptive reasoning schemes, Educ. Res. Eval. Int. J. Theor. Pract., 11(6), 651–589. Special issue: The Role of Research in Using Technology to Enhance Learning in Science. Guest Editors: Zacharias C. Zacharia and Constantinos P. Constantinou.
- Eryilmaz A., (2002), Effects of conceptual assignments and conceptual change discussions on students' misconceptions and achievement regarding force and motion, J. Res. Sci. Teach., 39(10), 1001–1015.
- Garnett P. J., (1996), Foundations of Chemistry, Australia: Longman Press.
- Garnett P. J. and Treagust D. F., (1990), Implication of research on student understanding of electrochemistry for improving science curricula and classroom practice, Int. J. Sci. Educ., 12(2), 259–262.
- Gay G., (2010). Culturally responsive teaching. New York: Teachers College Press.
- Haberman M., (1991), The pedagogy of poverty versus good teaching, Phi Delta Kappan, 73(4), 290–294.
- Hand B. and Treagust D. F., (1991), Student achievement and science curriculum development using a constructivist framework, Sch. Sci. Math., 91, 172–176.
- Honig M. and McDonald M., (2005). From promise to participation, New York: Robert Bowne Foundation.
- Hull G., (1997), Research with words: qualitative inquiry. Focus on Basics 1, No. A, Boston, MA: National Center for the Study of Adult Learning and Literacy.
- Ivarsson J., Schoult J. and Saljo R., (2002), Map reading versus mind reading: revisiting children's understanding of the shape of the earth, in Limon M. and Mason L. (ed.), Reconsidering conceptual change: issues in theory and practice, Dordrecht: Kluwer Academic Publishers, pp. 77–99.
- Kind V., (2004), Beyond Appearances: students' misconceptions about chemical education, 2nd edn, Durham University School of Education.
- Lindo E., (2006), The African–American presence in reading intervention experiments, Remedial and Special Education, 27(3), 148–153.
- Littky D., (2004), The big picture: education is everyone's business, Alexandria, VA: Association for Supervision and Curriculum Development.
- Lybeck L., Marton F., Stromdahl H. and Tulberg A., (1988). The phenomenography of the “mole concept” in chemistry, in Ramsden P. (ed.), Improving learning: new perspectives, London: Kogan Page, pp. 81–108.
- Marton F., (1984), Toward a psychology beyond the individual, in Lagerspetz K. and Niemi P. (ed.), Psychology in the 1990's, Amsterdam: North-Holland, pp. 45–72.
- Marton F. and Booth S., (1997), Learning awareness, Mahwah, NJ: Lawrence Erlbaum Associates Publishers.
- Marton F. and Tsui A. B. M., (2004). Classroom discourse and the space of learning, Mahwah, NJ: Lawrence Erlbaum.
- McCroskey J. C., (1992). An introduction to communication in the classroom, Edina, MN: Burgess International Group.
- McCroskey J. C. and Teven I., (1997), The relationship of perceived teacher caring with student learning and teacher evaluation, Commun. Educ., 46(1), 1–9.
- Michigan Department of Education (MDE), (2007). Science v.12.07 Grade level content expectations. Office of School Improvement. www.michigan.gov/mde.
- Morton T., (2012), Classroom talk, conceptual change and teacher reflection in bilingual science teaching, Teach. Teach. Educ. Int. J. Res. Stud., 28(1), 101–110.
- Mutegi J. W., (2010), The inadequancies of “science for all” and the neccessity and nature of a socially transformative curriculum approach for African American science education, J. Res. Sci. Teach., 48, 301–316.
- Nakhlen M. B. and Krajcik J. S., (1993), A protocol analysis of the influence of technology on students actions, verbal commentary, and thought process during the performance of acid base titrations, J. Res. Sci. Teach., 30(9), 1149–1168.
- National Commission on Teaching and America's Future, (1996), What matters most: teaching for America's future, New York: National Commission on Teaching and America's Future.
- National Research Council, (2002), Scientific research in education, Washington, DC: National Academies Press.
- Noam G., Biancarosa G. and Dechausay N., (2003), After-school education: approaches to an emerging field, Cambridge, Mass: Harvard Education Press.
- Noddings N., (1992), The challenge to care in the schools, New York, NY: Teachers College Press.
- Noddings N., (2005), What does it mean to educate the whole child? Educ. Leader., 63(1), 8–31.
- Noddings N., (2010), Moral education in an age of globalization, Educ. Philos. Theor., 42(4), 390–396.
- O'Connor E. and McCartney K., (2007), Examining teacher–child relationships and achievement as part of an ecological model of development, Am. Educ. Res. J., 44(2), 340–369.
- Orgill M., (2002), Phenomenography. Unpublished manuscript adopted for use in “Qualitative research methods in education” class (Educational Curriculum and Instruction 615). Purdue University, West Lafayette, IN.
- Peterson R., Treagust D. and Garnnet P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding, J. Res. Sci. Teach., 26(4), 301–314.
- Pintrich P. R., Marx R. W. and Boyle R. A., (1993), Beyond cold conceptual change: the role of motivational beliefs and classroom contextual factors in the process of conceptual change, Rev. Educ. Res., 63, 167–199.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66, 211–227.
- Renstrom L., (1988), Conceptions of Matter: A Phenomenographic Approach, vol. 69, Goteborg Sweden: Acta Universitatis Gothoburgensis.
- Ross B. and Munby H., (1991), Concept mapping and misconceptions: a study of high school students' understandings of acids and bases, Int. J. Sci. Educ., 13, 11–23.
- Ruiz-Primo M. A. and Furtak E. M., (2007), Exploring teachers' informal formative assessment/practices and students' understanding in the context of scientific inquiry, J. Res. Sci. Teach., 44(1), 57–84.
- Saljo R., (1988). Learning in educational settings: methods of inquiry, in Ramsden P. (ed.), Improving learning: new perspectives, London: Kogan Page.
- Sanders W. and Horn S., (1994), The Tennessee value-added assessment system (TVAAS): mixed-model methodology in educational assessment, J. Person. Eval. Educ., 8(3), 299–311.
- Schroeder C., Scott P., Tolson H., Huang T. and Lee Y., (2007), A meta-analysis of national research: effects of teaching strategies on student achievement in science in the United States, J. Res. Sci. Teach., 44(10) 1436–1460.
- Shavelson R., Phillip D., Towne L. and Feuer M., (2003), On the science of education design studies, Educ. Res., 32(1), 25–28.
- Sjostrom B., (2002), Applying phenomenography in nursing research, J. Adv. Nurs., 40(3), 339–345.
- Sungur S., Tekkaya C. and Geban Ö., (2001), The contribution of conceptual change texts accompanied by concept mapping to students' understanding of the human circulatory system, Sch. Sci. Math., 101(2), 91–101.
- Thagard P., (1992), Analogy, explanation and education, J. Res. Sci. Teach., 29(6), 537–544.
- Treagust D., (1988), Development and use of diagnostic tests to evaluate students' misconceptions in science, Int. J. Sci. Educ., 10(2), 159–169.
- Van Manen M., (2002), Researching the experience of pedagogy, Educ. Can., 42(3), 24–27.
- YouthBuild USA, (2011), Stories of transformation. Annual Report.
- Zoller U., (1990), Students' misunderstandings and alternative conceptions in college freshman chemistry (general and organic), J. Res. Sci. Teach., 27(10), 1053–1065.
| This journal is © The Royal Society of Chemistry 2013 |
