How does viewing one computer animation affect students' interpretations of another animation depicting the same oxidation–reduction reaction?
Deborah P.
Rosenthal
a and
Michael J.
Sanger
*b
aWashington State Community College, 710 Colgate Drive, Marietta, OH 45750, USA. E-mail: drosenthal@wscc.edu; Tel: +1 (740) 374-2147
bDepartment of Chemistry, Middle Tennessee State University, P.O. Box 68, Murfreesboro, TN 37132, USA. E-mail: michael.sanger@mtsu.edu; Fax: +1 (615) 898-5182; Tel: +1 (615) 904-8558
First published on 12th April 2013
Abstract
Two groups of students were shown unnarrated versions of two different particulate-level computer animations of varying complexity depicting the oxidation–reduction reaction of aqueous silver nitrate and solid copper metal; one group saw the more simplified animation first and the more complex animation second while the other group saw these animations in the reverse order. The goal of this study is to determine how viewing one of the animations affects the participants' subsequent explanations of the other animation. Viewing the more complex animation before the more simplified animation did not affect the participants' explanations of the more simplified animation, but did lead to a slight improvement in their abilities to write a balanced chemical equation of the oxidation–reduction reaction. Viewing the more simplified animation before viewing the more complex animation improved the participants' explanations of the more complex animation with respect to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the silver and nitrate ions, the 2
1 ratio of the silver and nitrate ions, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of the silver ions and the copper atoms, the electron transfer process, and writing a balanced equation for this reaction. This positive effect was attributed to the fact that the more simplified animation was easier to interpret since it depicted fewer objects on-screen moving around at the same time, and was therefore less confusing or distracting to the participants. Viewing the more simplified animation before the more complex animation negatively impacted their explanations of the source of the blue colour in the aqueous solution. This negative impact was attributed to the fact that the more simplified animation explicitly depicted the colour change and caused participants viewing the more complex animation to expect that animation to also explicitly depict this colour change.
1 reacting ratio of the silver ions and the copper atoms, the electron transfer process, and writing a balanced equation for this reaction. This positive effect was attributed to the fact that the more simplified animation was easier to interpret since it depicted fewer objects on-screen moving around at the same time, and was therefore less confusing or distracting to the participants. Viewing the more simplified animation before the more complex animation negatively impacted their explanations of the source of the blue colour in the aqueous solution. This negative impact was attributed to the fact that the more simplified animation explicitly depicted the colour change and caused participants viewing the more complex animation to expect that animation to also explicitly depict this colour change.
Introduction
Chemical education researchers interested in evaluating and improving students' abilities to visualize chemical phenomena at the particulate level have demonstrated that the use of computer animations depicting chemical processes at the particulate level can improve chemistry students' visualization skills (Williamson and Abraham, 1995; Russell et al., 1997; Sanger and Greenbowe, 2000; Sanger et al., 2000, 2001, 2007; Sanger and Badger, 2001; Ardac and Akaygun, 2004; Kelly et al., 2004; Velázquez-Marcano et al., 2004; Tasker and Dalton, 2006; Kelly and Jones, 2007, 2008; Gregorius et al., 2010a, 2010b; Rosenthal and Sanger, 2012, 2013a, 2013b; Williamson et al., 2012). Most of this research used these particulate-level computer animations as part of instructional interventions designed to improve students' conceptual understanding of chemical phenomena. This study is one of a growing number that have started to use these animations as part of the assessment process (Sanger et al., 2007; Naah and Sanger, 2012, 2013; Rosenthal and Sanger, 2012, 2013a, 2013b).Rosenthal and Sanger (2013a) compared the particulate explanations of an oxidation–reduction reaction involving copper metal and an aqueous silver nitrate solution from two groups of students who had viewed a chemical demonstration of this reaction and one of two different particulate-level computer animations of this reaction. The two animations depicted the same chemical reaction, but differed in the levels of complexity of the visual images used in these animations. The animation created by Michael J. Sanger (referred to as the ‘more simplified animation’) used a static camera angle, did not depict water molecules in solution, and depicted objects moving and colliding in a single plane. The animation created as part of the VisChem project (Tasker and Dalton, 2006), and referred to as the ‘more complex animation’, used a changing camera angle, depicted water molecules as part of the aqueous solution, and allowed objects to move in front of or behind each other. This study showed that students viewing the more simplified animation provided better explanations for eight different concepts related to the oxidation–reduction reaction compared to the students viewing the more complex animation. Students viewing the more simplified animation also provided more accurate balanced chemical equations for this reaction compared to the students viewing the more complex animation. Quotes from students in both groups suggested that those viewing the more complex animation underperformed compared to those viewing the more simplified animation because the more complex animation depicted extraneous information and either did not depict relevant information or depicted relevant information that was difficult for students to see due to the detrimental effects of the extraneous information.
In a follow-up study, Rosenthal and Sanger (2013b) compared how the order of viewing the two different animations of the copper–silver nitrate oxidation–reduction reaction affected the students' particulate-level explanations of this reaction. They found that students who viewed the more complex animation followed by the more simplified animation provided better explanations for seven concepts and provided more correct balanced chemical equations than those students who viewed the animations in the reverse order. Students who favoured showing the more complex animation followed by the more simplified animation believed that the more complex animation will get students' attention (by entertaining or confusing them), and then the more simplified animation will more clearly explain what is happening in the reaction, leading to improved learning. However, interpretation of the results from this study are complicated by the fact that many of the students' explanations were directly tied to the version of the animation they were viewing, and so the differences in their explanations may be an artefact of the last animation viewed and not necessarily the order in which the two animations were viewed.
One of the conclusions from this last study (Rosenthal and Sanger, 2013b) was that viewing the more simplified animation served as an instructional cue (Mayer and Gallini, 1990; Patrick et al., 2005; Mayer and Wittrock, 2009; Cook et al., 2011; Lin and Atkinson, 2011) that assisted students in interpreting the more complex animation. However, this assertion was not specifically tested in that study. The goal of the present study is to determine how viewing one of the animations affects the participants' subsequent explanations of the other animation.
Theoretical framework
When describing chemical phenomena, chemists often use three related but distinct representational levels—the macroscopic, particulate, and symbolic levels (Johnstone, 1993; Gilbert and Treagust, 2009; Johnstone, 2010; Talanquer, 2011). The macroscopic representation involves qualitative observations of chemical phenomena made using the five senses (colour changes, odours, heat changes, etc.), the particulate representation involves the behaviour of atoms, molecules, and ions involved in the chemical phenomena, and the symbolic representation involves the use symbols (numbers, mathematical formulas, chemical symbols and formulas, balanced equations, etc.) to represent more abstract concepts.The effectiveness of using computer animations of chemical processes at the particulate level is based on Mayer's cognitive theory of multimedia learning (Mayer, 2001), which was adapted from Paivio's dual-coding theory (Paivio, 1986) and Baddeley's model of working memory (Baddeley, 1986). Mayer's theory assumes that learners possess separate cognitive channels for processing visual (pictorial) and auditory (verbal) information, that learners have limited processing capabilities in each channel, and that learners engage in active learning by attending to relevant information, organizing this information into mental schema, and integrating this new knowledge with pre-existing knowledge.
Mayer's theory of multimedia learning incorporates cognitive load theory (Baddeley, 1986; Sweller, 1994; Sweller and Chandler, 1994), which assumes that learners have limited working memory and an unlimited long-term memory. If the cognitive load of the instructional lesson exceeds the limits of the learner's working memory, then learning will be hampered or diminished. There are two types of cognitive load that affect learning (Sweller, 1994; Sweller and Chandler, 1994). Intrinsic cognitive load is a property of the content to be learned; concepts that can be processed sequentially and independently of one another represent low intrinsic load, while concepts that must be processed simultaneously represent a higher intrinsic load. Extraneous cognitive load, on the other hand, is a function of how the instructional material is presented. Since the way a lesson is presented does not change the content to be learned, any extraneous cognitive load imposed by the way in which the lesson is presented uses up cognitive resources without improving learning (Lee et al., 2006).
Therefore, the goal of instructional design is to reduce extraneous cognitive load by manipulating verbal (text and narration) and pictorial information. For example, Mayer (2001) provides seven principles of multimedia design to minimize extraneous cognitive load based on the results of several educational research studies. Other researchers (Lee et al., 2006; Homer and Plass, 2010) have shown that adding iconic information to the symbolic visuals used in computer animations can improve student learning by minimizing extraneous cognitive load. Symbolic visuals use arbitrary representations to depict an object or concept, while iconic visuals use representations that are tied to the object or concept by surface-level relationships (e.g., when depicting an object being heated, using a slide bar with the word ‘Temperature’ above it represents symbolic visuals; showing the addition or removal of Bunsen burners below the object being heated or cooled represents iconic visuals). These researchers have shown that the positive effects of providing iconic information are largest when the students have low prior knowledge and when the concepts are highly complex (Lee et al., 2006; Kalyuga, 2007; Homer and Plass, 2010).
Methodology
Subjects
The sample consisted of 55 volunteer students (19 males and 36 females) enrolled in the same section of a second-semester introductory chemistry course intended for chemistry, biology, and health science majors and taught by the same chemistry instructor. Each participant was interviewed for 40–70 min after receiving classroom instruction on oxidation–reduction reactions and electrochemistry. The participants were randomly assigned via coin toss to one of two groups—one group (N = 26) viewed the more simplified animation before viewing the more complex animation while the other group (N = 29) viewed the more complex animation before the more simplified animation.Computer animations
The more simplified animation of the silver–copper reaction was created by the second author (Fig. 1a). This program was animated as two-dimensional such that when two objects approached each other, they were animated as colliding and bouncing off one another. The total viewing time for this animation is about 30 s; this animation was shown to the participants without narration. The animation shows copper-coloured circles in an organized pattern (copper metal) placed against a blue background (water). In the blue background, several silver-coloured circles with a ‘+’ symbol (silver ions) and an equal number of blue/red atom clusters with a ‘−’ symbol on it (nitrate ions) move freely. As the reaction occurs, two silver circles approach a copper circle, and a red ‘e−’ (electron) is transferred from the copper circle to each of the two silver circles. When the ‘e−’ are transferred, the copper circle becomes smaller and has a ‘2+’ symbol and each silver circle becomes larger and loses its ‘+’ symbol (electron transfer). The two silver circles stay attached to the copper metal and the copper ‘2+’ circle floats into the blue background. Each time this process occurs, the blue background becomes a darker blue colour.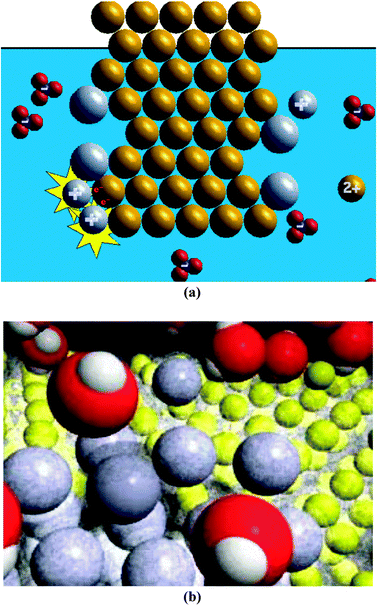 | ||
| Fig. 1 Screen shots for (a) the more simplified copper–silver animation created by Sanger and (b) the more complex copper–silver animation created by Tasker. | ||
The more complex animation of the silver–copper reaction (Fig. 1b) was created as part of the VisChem project (Tasker and Dalton, 2006) and was used with permission. This program was animated as three-dimensional such that when two objects approach each other, they were animated as moving in front of or behind one another. The total viewing time for this animation is also about 30 s; this animation was also shown to participants without its narrated audio track. This animation shows yellow spheres in an organized pattern (copper metal) placed against a black background. The yellow spheres (copper nucleus and core electrons) are surrounded by a transparent light grey sphere (valance electrons). Surrounding the yellow/light grey spheres is a large array of red/white atom clusters (water molecules). Mixed within the red/white shapes are grey spheres (silver ions) and blue/red atom clusters (nitrate ions). When a grey sphere comes in contact with the copper metal, a transparent light grey sphere enshrouds the grey sphere (electron transfer), and the grey/light grey sphere becomes attached to the copper metal. At a different time and spot, a yellow sphere loses its light grey outer sphere (electron transfer) and leaves the copper metal to mingle with the red/white shapes. Twice as many grey spheres attach to the copper metal as yellow spheres leave it. The red/white shapes appear to bring the grey sphere to the copper metal and then leave the grey sphere to interact with the other red/white shapes. The red/white shapes also appear to pull a yellow sphere in the copper metal away from it and away from its outer light grey sphere, and the yellow sphere (surrounded by red/white shapes) joins the red/white shapes. The blue/red clusters move among the red/white shapes, but neither changes during the animation.
Interview protocol
Participants' interpretations of the two animations were evaluated through the use of semi-structured interviews, which provided the flexibility for interviewers and participants to respond to emerging ideas and seek clarification (Borg and Gall, 1983). The interview questions for this study, which were generated from a list of conceptual and propositional knowledge statements, have been reported previously (Rosenthal and Sanger, 2012). For the first part of the interview, the participants were shown a live demonstration in which solid silver nitrate was dissolved in water and then a piece of solid copper metal was added to the solution and allowed to react. After the demonstration, the participants were asked to explain what they believed was happening in this reaction (what are the products, what does each chemical do as part of the reaction, where does the reaction occur, why does it occur, write a balanced equation for the reaction, etc.). For the second part, the participants were shown one of the animations and asked to explain how their perceptions of this reaction changed based on viewing the animation. In the third part, the participants were shown the other animation and asked to explain how their perceptions of this reaction changed based on viewing this animation. For Parts II and III, the participants were allowed to watch each animation as many times as they wanted during the interview, and were allowed to start and stop the animations to focus on a particular object or to see how two or more objects interacted. Half of the participants viewed the more complex animation first while the other half saw the more simplified animation first. In the fourth part, participants were asked to comment on the strengths and weaknesses of each animation.Data analysis
The participant–interviewer conversation for each interview was digitally recorded, and student-generated balanced equations were written on the question sheets used during the interview. The conversations were transcribed verbatim by the first author. These transcriptions were analysed and a summary of correct or incorrect conceptions was made for each participant that was confirmed or refuted by referring to the individual digital recordings. These summaries were combined for the two groups, and the aggregate data were compared for the two groups.The goal of this study was to determine how viewing one of the animations depicting the oxidation–reduction reaction affected the participants' interpretations of the other animation. To measure the effect of the more simplified animation on the participants' explanation of the more complex animation, we compared the responses of the participants from Part II who viewed the more complex animation (Group C) to the responses of participants from Part III who had viewed the more simplified animation followed by the more complex animation (Group SC). Similarly, to measure the effect of the more complex animation on the participants' explanation of the more simplified animation, we compared the responses of the participants from Part II who viewed the more simplified animation (Group S) to the responses of participants from Part III who had viewed the more complex animation and then the more simplified animation (Group CS).
These comparisons were made for a list of concepts previously identified by Rosenthal and Sanger (2012). For each of these concepts, each participant was given a numerical score and these values were compared using a one-way Analysis of Variance (ANOVA) with the animations viewed as the independent variable. For any of the concepts where participant scores could also be determined based on the chemical demonstration interview performed before viewing any animations, a one-way Analysis of Covariance (ANCOVA) was performed using the animation viewed as the independent variable and the demonstration score as the covariate.
Results and discussion
The statistical data used to determine how viewing the more complex animation affected the participants' explanations of the more simplified animation appear in Table 1. The data used to determine how viewing the more simplified animation affected the participants' explanations of the more complex animation are summarized in Table 2.| Concept | Animation effect | Covariate (demonstration) | ||||
|---|---|---|---|---|---|---|
| df | F value | p value | df | F value | p value | |
| a A statistical comparison could not be made because there was no variability in the participants' responses. b p < 0.05 corresponds to a significant difference between the responses of the two groups of participants. c p < 0.05 corresponds to a significant association between the dependent variable and the covariate. | ||||||
| Identifying water | 1,53 | 0.012 | 0.912 | — | — | — |
| Identifying nitrate ions | 1,53 | 0.113 | 0.738 | — | — | — |
| Absence of ion pairs | 1,52 | 2.233 | 0.141 | 1,52 | 0.147 | 0.703 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–nitrate ratio 1 silver–nitrate ratio |
1,52 | 2.891 | 0.095 | 1,52 | 0.698 | 0.407 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–copper ratio 1 silver–copper ratio |
1,52 | 1.756 | 0.191 | 1,52 | 2.630 | 0.111 |
| Electron transfer process | 1,52 | 0.114 | 0.737 | 1,52 | 67.843 | 0.000c |
| Atom/ion size changes | 1,53 | 3.343 | 0.073 | — | — | — |
| Source of the blue colour in solution | 1,52 | 0.061 | 0.806 | 1,52 | 21.982 | 0.002c |
| Water is not the driving forcea | — | — | — | — | — | — |
| Writing a balanced equation | 1,52 | 5.537 | 0.022b | 1,52 | 23.460 | 0.000c |
| Concept | Animation effect | Covariate (demonstration) | ||||
|---|---|---|---|---|---|---|
| df | F value | P value | df | F value | p value | |
| a p < 0.05 corresponds to a significant difference between the responses of the two groups of participants. b p < 0.05 corresponds to a significant association between the dependent variable and the covariate. | ||||||
| Identifying water | 1,53 | 0.370 | 0.546 | — | — | — |
| Identifying nitrate ions | 1,53 | 0.009 | 0.923 | — | — | — |
| Absence of ion pairs | 1,52 | 0.068 | 0.796 | 1,52 | 0.903 | 0.346 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–nitrate ratio 1 silver–nitrate ratio |
1,52 | 7.479 | 0.009a | 1,52 | 18.309 | 0.000b |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver–copper ratio 1 silver–copper ratio |
1,52 | 28.129 | 0.000a | 1,52 | 15.781 | 0.000b |
| Electron transfer process | 1,52 | 52.378 | 0.000a | 1,52 | 69.653 | 0.000b |
| Atom/ion size changes | 1,53 | 0.551 | 0.461 | — | — | — |
| Source of the blue colour in solution | 1,52 | 35.504 | 0.000a | 1,52 | 40.531 | 0.000b |
| Water is not the driving force | 1,52 | 0.012 | 0.914 | 1,52 | 0.643 | 0.426 |
| Writing a balanced equation | 1,52 | 9.638 | 0.003a | 1,52 | 3.608 | 0.063 |
Identifying water in the animations
During the chemical demonstration interviews, all 55 participants properly identified water as the clear colourless liquid, and all were able to draw water molecules as ‘H2O’, so there was no need to use these data as a covariate score. The more simplified animation does not depict the water molecules at the particulate level but instead depicts the presence of liquid water at the macroscopic level using a blue background. The more complex animation, on the other hand, depicted water as molecules with one red sphere and two white spheres, and this animation is dominated by the sheer presence of these red/white shapes.Over ninety per cent of the participants were able to identify the blue background in the more simplified animation as the depiction of water, and viewing the more complex animation did not appear to affect this interpretation, F(1,53) = 0.012, p = 0.912. The data showed that 92% of the S group and 93% of the CS group correctly identified the blue background as water.
Viewing the more simplified animation did not significantly affect the participants' interpretation of the red/white shapes in the more complex animation, F(1,53) = 0.370, p = 0.546. While 62% of the participants in the C group correctly identified the red/white shapes, only 54% of the participants in the SC group did. The most common mistake made when interpreting the red/white shapes was to identify them as nitrate ions.
Identifying nitrate ions in the animations
Both of the animations depicted nitrate ions as a cluster of one blue atom and three red atoms; the more simplified animation also had the blue atom of the cluster labelled with a ‘−’ symbol to show the charge of the nitrate ion. Viewing the more complex animation did not affect the participants' interpretation of the more simplified animation, F(1,53) = 0.113, p = 0.738 (92% of the S group and 90% of the CS group correctly identified the nitrate ions). Similarly, viewing the more simplified animation did not affect the participants' interpretation of the more complex animation either, F(1,53) = 0.009, p = 0.923 (45% of the C group and 46% of the SC group correctly identified the nitrate ions). The percentage of participants correctly identifying the nitrate ions was lower when viewing the more complex animation compared to the more simplified animation. Rosenthal and Sanger (2013a) noted that students were more likely to misinterpret the blue/red clusters in the more complex animation as hydrated copper ions or as the object making the aqueous solution turn blue as the reaction occurred.The absence of ion pairs in solution
All of the 55 participants recognized that solid silver nitrate in the chemical demonstration would dissolve in water and become aqueous. In the chemical demonstration interviews, 39 of the participants stated that the silver and nitrate ions would dissociate in water, 5 of the participants thought that ion-pairs would exist, and 11 of the participants weren't sure. The misconception that ionic compounds dissolve in water as neutral “molecules” or ion-pairs is well documented (Butts and Smith, 1987; Smith and Metz, 1996; Boo, 1998; Liu and Lesniak, 2006; Kelly and Jones, 2007; Tien et al., 2007; Kelly and Jones, 2008; Nyachwaya et al., 2011; Smith and Nakhleh, 2011; Rosenthal and Sanger, 2012; Naah and Sanger, 2013).Viewing the more complex animation did not affect the participants' interpretation of the more simplified animation, F(1,52) = 2.233, p = 0.141. Only 8% of the S group participants and 0% of the CS group participants suggested the presence of ion-pairs in solution. Viewing the more simplified animation did not affect the participants' interpretation of the more complex animation either, F(1,52) = 0.068, p = 0.796. About 59% of the C group participants and 62% of the SC group participants suggested that ion-pairs existed in solution. Rosenthal and Sanger (2012, 2013a, 2013b) provided student-generated quotes suggesting that when viewing the more complex animation, some students misinterpreted the white/red shapes as nitrate ions and would then misinterpret the interactions of the silver or copper ions and the red/white shapes (hydrated ions) as silver-nitrate or copper-nitrate ion pairs.
The ratio of silver ions to nitrate ions in solution
The more simplified animation shows an equal number of silver ions and nitrate ions when the animation begins. The more complex animation, on the other hand, shows many more silver ions compared to the number of nitrate ions. Since the nitrate ions are spectators and are not involved in the chemical reaction, the animator may have chosen to leave out these spectator ions in the interest of making an already complex animation easier to interpret.Only about two-thirds of the participants explicitly mentioned the silver ion to nitrate ion ratio during their interviews, and most of these comments focused on their interpretations of the images depicted in the animation being viewed and not on their personal beliefs regarding the correct ratio of these ions in solution. Therefore, for this comparison we decided to evaluate the chemical formula for silver nitrate provided by the participants in their self-generated balanced equations of this reaction.
Over ninety per cent of the participants viewing the more simplified animation recognized the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of silver and nitrate ions, and viewing the more complex animation did not appear to affect this interpretation, F(1,52) = 2.891, p = 0.095. The data showed that 92% of the participants in the S group and 100% of the participants in the CS group correctly identified the ratio of the silver and nitrate ions.
1 ratio of silver and nitrate ions, and viewing the more complex animation did not appear to affect this interpretation, F(1,52) = 2.891, p = 0.095. The data showed that 92% of the participants in the S group and 100% of the participants in the CS group correctly identified the ratio of the silver and nitrate ions.
On the other hand, viewing the more simplified animation appeared to improve the participants' interpretations of the ratio of silver to nitrate ions in the more complex animation, F(1,52) = 7.479, p = 0.009. While only 52% of participants in the C group correctly identified the silver-to-nitrate ratio, 92% of participants in the SC group correctly identified this ratio. A quote from a participant in the SC group also shows the positive effect viewing the more simplified animation had in his interpretation of silver-to-nitrate ratio in the more complex animation.
Interviewer: In this reaction [more simplified animation], how many nitrates and silvers?
Participant: Close to equal. In the other reaction [more complex animation], we did not think the nitrate was equal to the silver.
The ratio of silver ions and copper atoms in the reaction
In the more simplified animation, the reaction of two silver ions and one copper atom were animated to happen at the same time and at the same spot on the copper surface. Since copper metal is an electrical conductor, this reaction does not have to occur at the same spot on the surface—two silver ions can attach anywhere on the metal surface, each gaining an electron from the bulk metal, and causing the release of a single copper ion at another place on the surface. The more complex animation shows this more complex view of the oxidation–reduction process.Viewing the more complex animation did not affect the participants' interpretation of the more simplified animation, F(1,52) = 1.756, p = 0.191. Roughly 85% of the S group participants and 93% of the CS group participants recognized that two silver ions reacted with one copper atom in this reaction.
Viewing the more simplified animation, however, did affect the participants' interpretation of the more complex animation, F(1,52) = 28.129, p < 0.001. Only 24% of the participants in the C group correctly identified the reacting ratio of silver ions and copper atoms; however, that number was higher (85%) for the participants in the SC group. A quote from a participant in the SC group indicates that she recognized the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio after viewing the more simplified animation (as seen in her balanced chemical equation) but felt that the more complex animation was depicting an incorrect ratio that would have led to an incorrect balanced equation.
1 reacting ratio after viewing the more simplified animation (as seen in her balanced chemical equation) but felt that the more complex animation was depicting an incorrect ratio that would have led to an incorrect balanced equation.
Interviewer: Did this animation help you?
Participant: Yeah.
Interviewer: How?
Participant: The [silver–copper] ratio as two-to-one.
Interviewer: What do you think of the [more complex] animation?
Participant: Worse, because it did not show the [silver–copper] ratio. It showed one-to-one and screwed up the formula.
The electron transfer process for the reaction
There are four important concepts necessary to explain the electron transfer process in this reaction: Silver ions are reduced (gain electrons) during the reaction, each silver ion gains one electron during the reaction, copper atoms are oxidized (lose electrons) during the reaction, and each copper atom loses two electrons during the reaction. The participants were given a four-point score for their explanation of the electron transfer process (one point for each idea), both for their chemical demonstration interview and after viewing each animation. These values were compared using ANCOVA with the chemical demonstration scores serving as the covariate.Viewing the more complex animation did not affect the participants' explanations of the electron transfer process based on the more simplified animation, F(1,52) = 0.114, p = 0.737. The adjusted least squares means (corrected for the participants' chemical demonstration scores) were 3.1 out of 4 for the S group participants and 3.2 out of 4 for the CS group.
However, viewing the more simplified animation did improve the participants' explanations of the electron transfer process depicted by the more complex animation, F(1,52) = 52.378, p < 0.001. The adjusted least squares mean was 0.4 out of 4 for the C group participants, but was 1.6 out of 4 for the SC group participants. A quote from another participant in the SC group demonstrates the positive effect of seeing the more simplified animation on her interpretation of electron transfer process shown in the more complex animation. This participant articulates all four electron-transfer ideas after viewing the more simplified animation, but does not believe that she would have recognized these concepts after viewing only the more complex animation.
Participant: Each copper atom is losing two electrons. One of those would go to one silver, and one of those would go to another silver.
Interviewer: Now is that easy, can you see that in the [more complex] animation?
Participant: Not if we had not seen the first [more simplified] one, no. If someone had just said, ‘Hey we are going to see redox reactions today and had just shown me this one, I would be confused to all get-out, like ‘What are all those little spheres for?’
Size changes for the metal atoms and ions during the reaction
In the more simplified animation, the silver circle gets larger when the silver ion gains an electron to become a silver atom, and the copper circle gets smaller when the copper atom loses two electrons to become a copper(II) ion. In the more complex animation, the sizes of the yellow and dark grey spheres (representing the “core” electrons of the Cu2+ and Ag+ ions, respectively) stay the same—the neutral metal atoms are depicted by the solid sphere surrounded by a transparent light grey sphere of valence electrons. For this topic, participants were given a two-point score for their explanation of these size changes (one point for stating that the silver ion gets larger when it gains an electron, and one point for stating that the copper atom gets smaller when it loses two electrons).Almost all of the participants viewing the more simplified animation recognized the correct size changes, and viewing the more complex animation did not appear to affect this interpretation, F(1,53) = 3.343, p = 0.073. The adjusted least squares means were 1.8 out of 2 for the S group participants and 2.0 out of 2 for the CS group participants. Although fewer participants viewing the more complex animation correctly identified the size changes during the reaction, viewing the more simplified animation did not affect their interpretations of the more complex animation either, F(1,53) = 0.551, p = 0.461. The adjusted least squares means were 0.6 out of 2 for the C group participants and 0.7 out of 2 for the SC group participants.
The source of the blue colour in solution after the reaction
The more simplified animation showed the light blue background representing the water turning a darker blue colour after each oxidation–reduction event. The more complex animation, on the other hand, did not depict the solution colour or any changes to its colour. Participants in this study were given two points for recognizing that the aqueous solution turns blue because of the formation of Cu2+(aq) ions. They were given one point if they stated that the blue colour came from aqueous copper(II) nitrate, and no points if they suggested that the blue colour came from any other source.Most of the participants viewing the more simplified animation attributed the colour change of the solution to the formation of copper ions, and viewing the more complex animation did not appear to affect this interpretation, F(1,53) = 0.061, p = 0.806. The adjusted least squares mean were 1.6 out of 2 for the participants in the S group and 1.7 out of 2 for the participants in the CS group.
However, viewing the more simplified animation actually worsened the participants' explanations of the source of the blue colour depicted by the more complex animation, F(1,52) = 35.504, p < 0.001. The adjusted least squares mean was 0.8 out of 2 for the C group participants, but was −0.1 out of 2 for the SC group participants. Some of the SC participants who correctly identified copper ions as the source of the blue colour in the more simplified animation simply stated that they could not identify the source of the blue colour in the more complex animation.
Interviewer: What is causing the blue colour?
Participant: Copper coming off. Copper nitrate.
Interviewer: Copper coming off, or copper and nitrate together?
Participant: Good question. Well, in this photo it looks like with every copper [ion released] water gets bluer, so it does not look like it has anything to do with nitrate (more simplified animation).
Interviewer: Can you tell what is causing the blue [colour]?
Participant: No, I can't even tell it is changing to blue (more complex animation).
Most of the other SC participants who correctly identified the source of the blue colour in the more simplified animation believed that the blue atoms in the blue/red clusters (nitrate ions) were responsible for the blue colour in solution; some of these participants referred to these blue/red clusters as nitrate ions, while others called them ‘blue molecules’ or ‘darker water molecules’.
Interviewer: Based on this [more complex] animation, do you know what is causing the blue colour?
Participant: I didn't see a colour change. Well, the nitrate had some blue stuff.
Interviewer: Does this [more complex animation] explain the blue colour change?
Participant: I would assume the blue molecule in the animation is the blue in the water.
Interviewer: What is that blue thing with red?
Participant: That is the darker water molecule.
Interviewer: And this is what is causing…
Participant: The blue colour. But in this picture it didn't show the plus molecule [copper ion] coming out and the background getting darker, so I guess this molecule should show the water getting darker?
Water's role as a driving force for the reaction
The more simplified animation shows that the ions are completely dissociated in the aqueous solution but does not show water molecules interacting with these ions. The more complex animation, on the other hand, shows the hydration of the aqueous ions and the interactions of these ions with water molecules. Unfortunately, some participants viewed the water molecules in the more complex animation as taking an active role by pushing the silver ions to the metal surface to react and by pulling the copper ions out from the bulk metal into the solution (Tasker and Dalton, 2006; Rosenthal and Sanger, 2012). These participants incorrectly believed that water was acting as the driving force for this reaction, forcing it to occur.None (0%) of the participants in the S group or the CS group made statements indicating that water was forcing this reaction to occur. Unfortunately, since there was no variability in these participants' responses, no statistical comparisons could be made between the two groups. Still, it does appear that viewing the more complex animation did not affect the participants' ability to interpret water's role in forcing this reaction to occur.
Viewing the more simplified animation did not significantly affect the participants' interpretation of water's role in driving the reaction depicted in the more complex animation, F(1,52) = 0.012, p = 0.914. Approximately 61% of the participants in the C group and 66% of the participants in the SC group incorrectly suggested that the red/white shapes (water molecules) were forcing the reaction to occur by pushing the silver ions to the metal surface and pulling the copper ions away from the metal surface. Of the 61% of C group participants believing that the red/white shapes were driving the reaction, 42% recognized that the red/white shapes were water molecules and 19% believed that they were nitrate ions; for the SC group participants, these values were 38% and 28%, respectively.
Writing balanced chemical equations for the reaction
Near the end of Parts I–III of the interviews, each participant was asked to write a balanced chemical equation for the oxidation–reduction reaction between copper and silver nitrate. Each participant was given a 20-point score for their balanced equation based on the chemicals present in the equation (formulas, charges, states of matter), the stoichiometric ratios, and atom and charge balance. These values were used to perform ANCOVA calculations, using the chemical demonstration score as the covariate.Viewing the more complex animation did improve the participants' ability to write a balanced equation after viewing the more simplified animation, F(1,52) = 5.537, p = 0.022. The adjusted least squares mean was 17.7 out of 20 for the S group participants, but was 19.3 out of 20 for the CS group participants. Similarly, viewing the more simplified animation also improved the participants' ability to write a balanced equation after viewing the more complex animation, F(1,52) = 9.638, p = 0.003. The adjusted least squares mean was only 14.9 out of 20 for the C group participants, but was 17.9 out of 20 for the SC group participants. Although none of the SC participants made comments describing how viewing the more complex animation after the more simplified animation improved their ability to write a balanced equation for this reaction, a few CS participants indicated that viewing the more simplified animation after seeing the more complex animation helped them write a more correct balanced equation for this reaction.
Interviewer: Does this [more simplified animation] make you change your equation? How do you know to put ‘2’ in front of silver nitrate?
Participant: Animation.
Interviewer: Balanced?
Participant: Much more confident.
Interviewer: [So it] Helped with [the] balanced equation?
Participant: Yes.
Interviewer: Coefficients?
Participant: Yes. Better than before.
Interviewer: What do you think of this animation?
Participant: I liked it. The first one [more complex animation] was neat. Seeing it gave you a better idea of what was going on visually. This one [more simplified animation] gave a better idea of what was going on chemically.
Additional participant comments
In addition to the statistical comparisons performed in this study, the participants' comments were analysed to look for trends in their beliefs about the relative usefulness of these two animations in helping them understand this chemical reaction. Most of the comments from the CS participants indicated that they found the more simplified animation easier to understand because it was simpler and showed fewer objects on-screen moving around at the same time.Participant: This one [more simplified animation] helps me better.
Interviewer: Why?
Participant: There is not as much going on. The other one [more complex animation] kept moving. It was a lot prettier, the other one. It was more entertaining. It was in 3-D. I like to look at 3-D ‘cause that is how I think. I like this one better ‘cause it is simpler. You can tell the copper comes off [and] makes the water blue. You can tell one copper to two silver. For learning purposes, this one is better.
Similar comments from several SC participants indicated that they too found the more simplified animation easier to understand because it showed fewer objects on-screen at the same time and was therefore less distracting.
Participant: I do not think this one [more complex animation] is clear. There is so much going on that I can't process it. Like, I can't pick apart all that is moving, and that it being 3-D… I am a little more confused than [with] the last one.
Interviewer: What is confusing about it?
Participant: …So many elements are involved and it is close-up too, that it hard to determine stuff from each other.
Interviewer: Is it just that it is so distracting ‘cause so much is going on?
Participant: M'hm. I think so.
In addition, several SC participants indicated that they would not have been able to make sense of the more complex animation if they had not seen the more simplified animation first.
Interviewer: What are your general thoughts (referring to the more complex animation)?
Participant: If we had not talked about it before, I would have no idea what is going on. I can see, like, the silver going to the copper and the copper coming out, but it is a bunch of jumbling on the screen.
Interviewer: So what helped?
Participant: The first [more simplified] animation. If I had not seen the first animation, I would have no idea what was going on.
Participant: If it [more complex animation] is supposed to represent the same thing, I think the first one [more simplified animation] is way easier. This looks like a blood stream or something. I can't just watch it and tell you what is happening… .
Interviewer: Can you tell me how many coppers are reacting with how many silvers?
Participant: I couldn't. Yeah, just one of them came up and the nitrates [red/white shapes] were in the way too much, and you can barely see the copper leave, and I wouldn't have even caught that if I hadn't seen the first animation first that explained it.
Conclusions
Viewing the more complex animation did not appear to have an effect on the participants' explanations of the information depicted by the more simplified animation. However, viewing the more complex animation did lead to a slightly improved score for the balanced chemical equation of this reaction, suggesting that those participants who viewed the more complex animation followed by the more simplified animation were better at writing a balanced equation for this reaction than participants who viewed only the more simplified animation. Comments from students in a previous study involving these animations (Rosenthal and Sanger, 2013b) suggested that the advantage of showing the more complex animation before the more simplified animation is that the more complex animation will get students' attention and this will prepare them to attend to the information presented by the more simplified animation. In this way, the more complex animation can serve to activate students' schema in their long-term memory and this would improve student learning by connecting the new material to relevant stored information (Mayer and Gallini, 1990; Patrick et al., 2005; Mayer and Wittrock, 2009; Cook et al., 2011; Lin and Atkinson, 2011).On the other hand, viewing the more simplified animation did have an effect on the participants' explanations of the information depicted by the more complex animation. Specifically, compared to participants who viewed the more complex animation only, participants who viewed the more simplified animation before the more complex animation were better at recognizing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the silver and nitrate ions, recognizing the 2
1 ratio of the silver and nitrate ions, recognizing the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reacting ratio of the silver ions and the copper atoms, explaining the electron transfer process, and writing a balanced equation for this reaction. These results are consistent with the results of a previous study (Rosenthal and Sanger, 2013b) suggesting that the more simplified animation served as an instructional cue (Mayer and Gallini, 1990; Patrick et al., 2005; Mayer and Wittrock, 2009; Cook et al., 2011; Lin and Atkinson, 2011) that helped students interpret the more complex animation.
1 reacting ratio of the silver ions and the copper atoms, explaining the electron transfer process, and writing a balanced equation for this reaction. These results are consistent with the results of a previous study (Rosenthal and Sanger, 2013b) suggesting that the more simplified animation served as an instructional cue (Mayer and Gallini, 1990; Patrick et al., 2005; Mayer and Wittrock, 2009; Cook et al., 2011; Lin and Atkinson, 2011) that helped students interpret the more complex animation.
There are two possible explanations for the benefits of viewing the more simplified animation before the more complex animation. The first explanation is that since the more simplified animations showed fewer objects on-screen at the same time, the extraneous cognitive load of this animation was lower than for the more complex animation (Sweller, 1994; Sweller and Chandler, 1994). Participants from both groups commented that the more simplified animation was easier to interpret because it depicted fewer objects on-screen moving around at the same time, and was therefore less confusing or distracting. Several participants in the SC group also made comments suggesting that they would not have been able to interpret the more complex animation if they had not first seen the more simplified animation. These results are consistent with Mayer's coherence principle (2001), which states that students learn better when extraneous material is excluded rather than included.
The second explanation of the benefits of viewing the more simplified animation before the more complex one is that the more simplified provides additional, iconic, information that helped students interpret the symbolic information presented in the more complex animation. Participant comments from this study and previous ones using these animations (Rosenthal and Sanger, 2012, 2013a, 2013b) showed the depicting the ion charges, the electrons (as red ‘e−’ symbols), and colour changes in the blue background helped students better interpret the more simplified animation compared to the more complex animation. The improved learning based on the addition of iconic information in the more simplified animation to the symbolic information used in both animations is consistent with the results of previous research (Lee et al., 2006; Homer and Plass, 2010). Unfortunately, this study cannot determine which of these two effects (or both) were responsible for the improved learning of the participants in this study.
Viewing the more simplified animation had a negative effect on the participants' explanations of the source of the blue colour in the more complex animation. Many of these participants stated that they could not see the colour change depicted in the more complex animation while others attributed the blue colour to the blue nitrogen atom in the blue/red cluster of the nitrate ion. It appears that by explicitly depicting the changes in the blue colour of the aqueous solution, the more simplified animation convinced some of the participants that the more complex animation would also be explicitly depicting this colour change. Therefore, these participants were confused when they did not see the colour change depicted or interpreted the blue atom in the nitrate ions as being this explicit depiction of the colour change.
This study, and the conclusions based on the results of this interview study, is limited by the fact the computer animations in this study were used without narration, contrary to the intentions of the creators of both animations. The choice to exclude narrations was made by the authors because we wanted to see whether the participants could interpret the animations without narration or further explanation since students could watch these animations on a computer without sound or without viewing previous multimedia lessons that explain the objects depicted in these animations. Therefore, these results might have been different if the participants had viewed the narrated animations. Mayer's multimedia theory of learning (2001) assumes that learners use two cognitive channels for processing visual and auditory information; by removing the narrated audio tracks, this study prevented students from using their auditory processing channel. This likely increased the extraneous cognitive load (Sweller, 1994; Sweller and Chandler, 1994) of both animations by limiting the processing channels available for learning. Therefore, it is possible that the improved learning might occur when viewing the narrated version of the more complex animation, and this might cause the generally positive effect of viewing the more simplified animation on the participants' interpretation of the more complex animation to disappear or at least be diminished.
Several implications for animation designers and users can be drawn from the results of this study and previous ones using the same animations (Rosenthal and Sanger, 2012, 2013a, 2013b). These studies have shown that students can have difficulty interpreting animations shown without narration, demonstrating the importance of quality narration to explain what is being depicted in a computer animation. Therefore, animation designers need to carefully plan and evaluate the effectiveness of the narration accompanying any animation, and if narrations are missing then the users (instructors) may need to create narrations of their own. These studies also showed that student learning was improved when students viewed the more simplified animation (alone or in addition to the more complex animation). The benefit of viewing the more simplified animation appears to come from showing less extraneous information (water molecules) that might distract students' attention from the learning process and in including iconic information (ion charges, electrons, changes in the background colour, etc.) along with symbolic information. Animation designers should avoid the inclusion of extraneous information in animations that is not relevant to the learning process, and should include relevant iconic imagery in animations along with the symbolic imagery typically used in animations.
Notes and references
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change, J. Res. Sci. Teach., 41, 317–337.
- Baddeley A. D., (1986), Working memory, Oxford: Oxford University Press.
- Boo H. K., (1998), Students' understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35, 569–581.
- Borg W. R. and Gall M. D., (1983), Educational Research, 4th edn, New York: Longman, pp. 441–443.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17, 192–201.
- Cook M., Wiebe E. and Carter G., (2011), Comparing visual representations of DNA in two multimedia presentations, J. Educ. Multimedia Hypermedia, 20, 21–42.
- Gilbert J. K. and Treagust D. (ed.), (2009), Multiple representations in chemical education, Dordrecht: Springer-Verlag.
- Gregorius R. Ma., Santos R., Dano J. B. and Gutierrez J. J., (2010a), Can animations effectively substitute for traditional teaching methods? Part I: preparation and testing of materials, Chem. Educ. Res. Pract., 11, 253–261.
- Gregorius R. Ma., Santos R., Dano J. B. and Gutierrez J. J., (2010b), Can animations effectively substitute for traditional teaching methods? Part II: potential for differentiated learning, Chem. Educ. Res. Pract., 11, 262–266.
- Homer B. D. and Plass J. L., (2010), Expertise reversal for iconic representations in science visualizations, Instr. Sci., 38, 259–276.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70, 701–704.
- Johnstone A. H., (2010), You can't get there from here, J. Chem. Educ., 87, 22–29.
- Kalyuga S., (2007), Expertise reversal effect and its implications for learner-tailored instruction, Educ. Psychol. Rev., 19, 509–539.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students' explanations, J. Sci. Educ. Technol., 16, 413–429.
- Kelly R. M. and Jones L. L., (2008), Investigating students' ability to transfer ideas learned from molecular animations to the dissolution process, J. Chem. Educ., 85, 303–309.
- Kelly R. M., Phelps A. J. and Sanger M. J., (2004), The effects of a computer animation on students' conceptual understanding of a can-crushing demonstration at the macroscopic, microscopic, and symbolic levels, Chem. Educ., 9, 184–189.
- Lee H., Plass J. L. and Homer B. D., (2006), Optimizing cognitive load for learning from computer-based science simulations, J. Educ. Psychol., 98, 902–913.
- Lin L. and Atkinson R. K., (2011), Using animations and visual cueing to support learning of scientific concepts and processes, Comput. Educ., 56, 650–658.
- Liu X. and Lesniak K., (2006), Progression in children's understanding of the matter concept from elementary to high school, J. Res. Sci. Teach., 43, 320–347.
- Mayer R. E., (2001), Multimedia Learning, New York: Cambridge University Press.
- Mayer R. E. and Gallini J., (1990), When is an illustration worth ten thousand words?, J. Educ. Psychol., 82, 715–727.
- Mayer R. E. and Wittrock M. C., (2009), Problem solving, in Alexander P. A. and Winne P. H. (ed.), Handbook of Educational Psychology, 2nd edn, New York: Routledge, pp. 287–303.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13, 186–194.
- Naah B. M. and Sanger M. J., (2013), Investigating students' understanding of the dissolving process, J. Sci. Educ. Technol., 22, 103–112.
- Nyachwaya J. M., Mohamed A.-R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Paivio A., (1986), Mental representations: a dual coding approach, New York: Oxford University Press.
- Patrick M. D., Carter G. and Wiebe E., (2005), Visual representations of DNA replication: middle grades students' perceptions and interpretations, J. Sci. Educ. Technol., 14, 353–365.
- Rosenthal D. P. and Sanger M. J., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction, Chem. Educ. Res. Pract., 13, 471–483.
- Rosenthal D. P. and Sanger M. J., (2013a), Comparing students' explanations of an oxidation–reduction reaction based on two computer animations of varying complexity, J. Chem. Educ., under review.
- Rosenthal D. P. and Sanger M. J., (2013b), How does the order of viewing two computer animations of the same oxidation–reduction reaction affect students' particulate-level explanations?, in Suits J. P. and Sanger M. J. (ed.), Pedagogic Roles of Animations and Simulations in Chemistry Courses, Washington, DC: American Chemical Society, in press.
- Russell J. W., Kozma R. B., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74, 330–334.
- Sanger M. J. and Badger II S. M., (2001), Using computer-based visualization strategies to improve students' understanding of molecular polarity and miscibility, J. Chem. Educ., 78, 1412–1416.
- Sanger M. J., Brecheisen D. M. and Hynek B. M., (2001), Can computer animations affect college biology students' conceptions about diffusion and osmosis?, Am. Biol. Teach., 63, 104–109.
- Sanger M. J., Campbell E., Felker J. and Spencer C., (2007), Concept learning versus problem solving: does particle motion have an effect?, J. Chem. Educ., 84, 875–879.
- Sanger M. J. and Greenbowe T. J., (2000), Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies, Int. J. Sci. Educ., 22, 521–537.
- Sanger M. J., Phelps A. J. and Fienhold J., (2000), Using a computer animation to improve students' conceptual understanding of a can-crushing demonstration, J. Chem. Educ., 77, 1517–1520.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73, 233–235.
- Smith K. C. and Nakhleh M. B., (2011), University students' conceptions of bonding and melting and dissolving phenomena, Chem. Educ. Res. Pract., 12, 398–408.
- Sweller J., (1994), Cognitive load theory, learning difficulty and instructional design, Learn. Instr., 4, 295–312.
- Sweller J. and Chandler P., (1994), Why some material is difficult to learn, Cogn. Instr., 12, 185–233.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Tien T. L., Teichert A. M. and Rickey D., (2007), Effectiveness of a MORE laboratory module in prompting students to revise their molecular-level ideas about solutions, J. Chem. Educ., 84, 175–181.
- Velázquez-Marcano A., Williamson V. M., Ashkenazi G., Tasker R. and Williamson K. C., (2004), The use of video demonstration and particulate animation in general chemistry, J. Sci. Educ. Technol., 13, 315–323.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32, 521–534.
- Williamson V. M., Lane S. M., Gilbreath T., Tasker R., Ashkenazi G., Williamson K. C. and Macfarlane R. D., (2012), The effect of viewing order of macroscopic and particulate visualizations on students' particulate explanations, J. Chem. Educ., 89, 979–987.
| This journal is © The Royal Society of Chemistry 2013 |
