Reasoning using particulate nature of matter: An example of a sociochemical norm in a university-level physical chemistry class
Nicole
Becker†
*a,
Chris
Rasmussen
b,
George
Sweeney
c,
Megan
Wawro
d,
Marcy
Towns
e and
Renee
Cole
f
aClemson University, Department of Chemistry, Clemson, South Carolina 29634-0973, USA. E-mail: nicolembecker@gmail.com
bDepartment of Mathematics and Statistics, San Diego State University, 5500 Camanile Drive, San Diego, California 92182-7720, USA
cSanta Ana College Department of Mathematics, Santa Ana, California, USA
dVirginia Tech, Department of Mathematics, Blacksburg, Virginia, USA
ePurdue University, Department of Chemistry, West Lafayette, Indiana, USA
fUniversity of Iowa, Department of Chemistry, Iowa City, Iowa, USA
First published on 16th November 2012
Abstract
In college level chemistry courses, reasoning using molecular and particulate descriptions of matter becomes central to understanding physical and chemical properties. In this study, we used a qualitative approach to analyzing classroom discourse derived from Toulmin's model of argumentation in order to describe the ways in which students develop particulate-level justifications for claims about thermodynamic properties. Our analysis extends the construct of sociomathematical norms to a chemistry context in order to describe disciplinary criteria for reasoning and justification, which we refer to as sociochemical norms. By examining how whole class and small group discussions shape norms related to reasoning, we provide suggestions for teaching practices in inquiry-oriented settings.
Introduction
Participating in collaborative discourse in which students construct and defend their positions has been suggested as one way to support student conceptual understanding of science (e.g., Asterhan and Schwarz, 2007; Sampson and Clark, 2009; Osborne, 2010; Zohar and Nemet, 2002). Engaging students in classroom discourse offers opportunities for students to participate in the construction of joint understandings, to negotiate relationships between different types of evidence, and to practice making evidence-based claims about science content (Duschl and Osborne, 2002; Osborne, 2010). By interacting with peers, students may challenge one another to articulate their reasoning, to negotiate meanings of terminology or symbolic representations, and to elaborate what counts as evidence in various contexts, which may in turn support students' individual construction of knowledge (Asterhan and Schwarz, 2007).One inquiry-oriented approach for university-level chemistry courses that provides students with opportunities to engage in a collaborative discourse is the Process Oriented Guided Inquiry Learning (POGIL) approach. Since 2003, the POGIL project has fostered collaborative learning environments for chemistry courses by developing and disseminating instructional materials and providing instructor support (Moog, 2006; Process Oriented Guided Inquiry Learning, 2012; Spencer et al., 2003). POGIL instructional materials, including small group learning activities, are currently available for a number of courses ranging from introductory chemistry to upper division courses such as physical chemistry (Moog, 2006; Spencer et al., 2003). Descriptions of POGIL implementations have been provided for a variety of institutional contexts (Farrell et al., 1999; Hanson and Wolfskill, 1998, 2000; Spencer, 1999) and a number of student learning gains as a result of participation have been reported (Eberlein et al., 2008; Hanson and Apple, 2004; Lewis and Lewis, 2005).
Workbook materials for physical chemistry are structured to develop proficiency with mathematical manipulations and derivations and to promote discussion of chemistry concepts (Spencer et al., 2004). As such, questions included in these materials regularly prompt students to analyse data or explain observations. For instance, one question from a POGIL workbook unit on entropy (referred to as a critical thinking question) asks students to “Use a grammatically correct English sentence to explain the meaning of the derivative  ” (Spencer et al., 2004, p. 77). Such questions provide a context for the class to articulate understandings and to negotiate meanings of terms and symbols.
” (Spencer et al., 2004, p. 77). Such questions provide a context for the class to articulate understandings and to negotiate meanings of terms and symbols.
Though it is widely believed that inquiry-oriented instructional approaches such as POGIL have the potential to improve student content learning, few studies have examined how specific aspects of the classroom social environment contribute to student learning. Drawing on psychological perspectives, the majority of studies related to student learning of physical chemistry and POGIL instructional approaches have been designed and analysed in reference to individual student achievement. Complementary work that draws on sociological perspectives to analyse small group and whole class discourse is needed in order to inform the design of classroom learning environments and to inform the ways in which instructors can support student reasoning.
Using a qualitative approach to analysing classroom discourse, our research examines how classroom norms (sociological constructs) are established as the basis for student reasoning. Specifically, we explore the ways in which students develop justifications for claims about thermodynamic properties, and we describe how these ways of reasoning are shaped by whole class and small group discussions. That is, we describe how these ways of reasoning about chemistry concepts are constituted through classroom interaction.
Background
Theoretical perspective: social and sociochemical norms
The theoretical perspective that frames our research derives from social constructivist views of learning (Vygotsky, 1978) and the emergent perspective advanced by Cobb and Yackel (1996). In the emergent perspective, the classroom microculture that is established through interactions is considered to be an emergent phenomenon through which meanings are continuously re-negotiated through the course of teacher and students' interactions (Yackel and Cobb, 1996). Individual learning is viewed as inseparable from the social contexts in which it occurs, and as such, examining collaborative activity is critical to understanding how students learn (Cobb et al., 2001).A key notion from the emergent perspective is that collective classroom progress is enabled and constrained by the normative ways of reasoning and interacting that develop within the class. Normative ideas, that is ways of acting and interacting which become routine, may include ways of arguing, acting, and justifying which begin to function as though shared by the classroom community (Cobb et al., 2001). The phrases “taken-as-shared” and “function as-if-shared” have been used to refer to normative ideas in order to emphasize that although some jointly held meaning guides classroom interactions, these ideas may not be accurate depictions of every individual student's understandings (Cobb et al., 2001; Rasmussen and Stephan, 2008).
Our use of the term “sociochemical norm” is an adaptation of Yackel and Cobb's (1996) seminal work on sociomathematical norms to the undergraduate chemistry classroom. Sociomathematical norms are specific disciplinary criteria for more general social norms related to classroom discourse in mathematics. For example, within inquiry-oriented classrooms the following social norms often regulate discourse: students explain their reasoning, students listen to and try to make sense of other students' reasoning, and students indicate agreement or disagreement with other's reasoning. These particular social norms have little to do with the fact that the subject of instruction is mathematics. The class may have just as well been studying chemistry, mechanical engineering, or genetics. These norms, however, are critical parts of the classroom environment in that they shape how participants interact and contribute to the emerging discourse.
Sociomathematical norms, in contrast, refer to normative aspects of the classroom discourse that are specifically related to the fact that the subject of study was mathematics. For example, the specifics for what constitutes a mathematically different solution, an elegant mathematical solution, an efficient mathematical solution, and what constitutes an acceptable justification are all examples of sociomathematical norms (Yackel and Cobb, 1996; Yackel et al., 2000). To clarify, the expectation that one is to explain one's reasoning falls within an analysis of social norms, but the criterion for what constitutes an acceptable explanation is particular to the discipline.
Thus, we consider sociochemical norms to be criteria that regulate classroom discourse that are particular to the study of chemistry. For example, sociochemical norms shape student views of what counts as appropriate justification in chemistry, how different types of representations should be interpreted, and what counts as a “good” explanation in chemistry.
Two theoretical points regarding social and sociochemical norms are in order. First, social and sociochemical norms are not rules the instructor sets forth in the syllabus or states in class. Instead, social and sociochemical norms are the patterns of actual discourse constituted through on-going interactions. Second, social and sociochemical norms are constructs for which classroom discourse is the focus of analysis. Such collective-level patterns of interaction provide a basis by which meaningful individual learning can occur. While a significant body of literature has explored how sociomathematical norms emerge and contribute to classroom learning (e.g., Rasmussen et al., 2003; Yackel et al., 2000), to date, no work has explored how sociochemical norms shape learning in chemistry contexts.
Argumentation as a lens for classroom reasoning
In order to examine social and sociochemical norms in an inquiry-oriented physical chemistry class, we use classroom argumentation as a lens for analysing collaborative reasoning. Argumentation plays a critical role in the development of new knowledge (Kuhn, 2010). For instance, experimental evidence is particularly important to the construction of scientific theory, but in order to use experimental data to support a theory, scientists must provide explicit links as to the relevance of the data to the claim, and qualify the relationship with experimental settings and conditions under which the relationship is valid (Jiménez-Aleixandre and Erduran, 2007; Lawson, 2009; Toulmin, 1958). Frequently, scientific arguments consider multiple explanations for particular phenomena and as a result alternative arguments and relevant supporting or contradictory evidence may be considered as part of an argument (Lawson, 2009).Within the scientific community, arguments may be evaluated by means of written reviews (for example, peer-review of publications) and discourse such as that which occurs in academic seminars or presentations (Driver et al., 2000; Osborne, 2010). In this sense, scientific arguments are dialogical events between individuals or groups within the scientific community in which each offers justification for their views and provides counterarguments for oppositional views (Kuhn, 2010; Toulmin, 1958).
Argumentation in classroom contexts
In lecture settings, the potential for students to engage in the process of scientific argumentation infrequently occurs (Lemke, 1990; Osborne and Patterson, 2011). In such settings, students are more often expected to construct explanations involving causal accounts of observable phenomena. This type of explanations is the “bread and butter” of classroom discourse as students learning science are often expected to be able to reconstruct canonical explanations as evidence of their understanding of scientific concepts.The term argumentation, in contrast, implies a tentative claim that is supported by relatively certain grounds (Osborne and Patterson, 2011). Discipline-specific criteria, rather than logical soundness, frame the evaluation of an argument's validity. In this sense, argumentation refers to the social process through which reasoning takes place, while an argument is the outcome of the reasoning process (Jiménez-Aleixandre and Erduran, 2007; Kuhn and Udell, 2003; Osborne and Patterson, 2011). To engage in argumentation, individuals must attempt to “adjust their intentions and interpretations by verbally presenting the rationale of their actions” (Krummheuer, 1995, p. 229). The potential for this type of argumentation occurs more often in inquiry-oriented classrooms, such as the POGIL classroom that forms the setting for this study, than in traditional lecture classrooms (Lemke, 1990; Osborne and Patterson, 2011). In such classrooms, students are provided the space in which to discuss concepts with peers, to articulate their reasoning, and to make sense of the reasoning of others (Moog and Spencer, 2008).
Because of the importance of argumentation to scientific fields, it has been advocated that a key role of the science classroom should be to prepare students to enter this discourse (Bricker and Bell, 2008; Ford and Forman, 2006). Part of the rationale for having science classrooms introduce students to practices of scientific argumentation is that “understanding norms of scientific argumentation can lead students to understand the epistemological bases of scientific practice” (Sandoval and Millwood, 2008, p. 71). Furthermore, by modeling appropriate forms of arguments and providing space for students to practice constructing their own arguments, instructors have the potential to support students' developing ability to form scientific arguments about science concepts (Bricker and Bell, 2008; Kuhn, 2010).
The term collective argumentation has been used to refer to the collaborative meaning making process that typically occurs in classroom contexts as this term highlights the fact that in classroom contexts, arguments are most often constructed by multiple participants (Forman et al., 1998; Krummheuer, 1998; Yackel, 2002). In these settings, arguments are seldom elaborated in a linear fashion. In collective argumentation, disputes and requests for clarification among participants in a collaborative setting may lead to corrections, elaborations, revisions, and retractions from the original argument (Yackel, 2001). In the course of group activity, students may put forth claims without explicit justification, assuming that the basis for their reasoning is common knowledge for the group (Erduran et al., 2004). In such instances, claims may be supported with warrants and backings only as challenged. This format of argumentation has been referred to as informal argumentation and is the type of reasoning that is jointly constituted by participants as they engage in everyday tasks (Toulmin, 1958).
Toulmin's model of argumentation
Toulmin's (1958) model of argumentation (Fig. 1) describes the structure of argumentation as it occurs in a variety of domains. According to Toulmin's (1958) model (see Fig. 1), an argument is typically comprised of a series of statements, each of which plays a different role in the emerging argument's structure. Field-invariant features of arguments (those that are that are common across domains) include claims, data, warrants, and backings. A claim is typically a statement that is supported with evidence. The data for the argument is then the evidence that supports the claim. In classroom settings, evidence may take a variety of forms, ranging from factual information to procedures. A warrant may also be provided that articulates the relationship between the claim and the data. In addition to these core components, backings, qualifiers, and rebuttals may also be present. A backing may explain why the warrant is valid, and the qualifier may specify the conditions under which the argument would be valid. Rebuttals can address instances in which the warrant does not have the necessary authority, or instances in which the claim is not valid (Toulmin, 1958).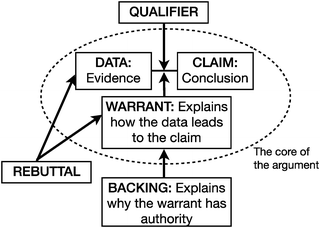 | ||
| Fig. 1 Toulmin's model of argumentation. | ||
It is important to note that the function played by a particular statement in a collaboratively constructed argumentation depends on the specific context of the discussion. Yackel (2001) noted that “what constitutes data, warrants, and backing is not predetermined but is negotiated by the participants as they interact” (p. 7).
Toulmin's (1958) model of argumentation has been used in a number of science contexts that examine how students construct scientific arguments in social settings (Erduran et al., (2004); Jiménez-Aleixandre and Erduran, 2007; Osborne, 2010). In mathematics education research, Toulmin’s (1958) model has been used to document collective argumentation and analyse evolving ideas in mathematics classroom contexts (Inglis et al., 2007; Stephan and Rasmussen, 2002; Weber et al., 2008). Similar models of argumentation have been used in order to relate classroom dynamics to reasoning (Berland and Lee, 2012).
Argumentation and epistemic criteria
Socially-established epistemic criteria for the evaluation of knowledge play a large role in shaping argumentation practices within a domain (Driver et al., 2000; Jiménez-Aleixandre and Erduran, 2007). While the core components of an argument (claim, data and warrant) can be found in reasoning across many domains, what counts as appropriate evidence in a particular domain is field-dependent (Jiménez-Aleixandre and Erduran, 2007).As previously described, we use the term sociochemical norms to refer to epistemic criteria related to what constitutes appropriate forms of evidence and reasoning within chemistry contexts. In classroom settings, sociochemical norms related to students' understanding of what constitutes a valid argument or appropriate use of evidence in chemistry contexts frame the ways in which students coordinate evidence to support claims about chemical and physical properties. While such epistemic criteria are not explicit components in Toulmin's model, they may be reflected in claim-data-warrant patterns across multiple classroom sessions. Our study focuses on identifying these patterns in classroom argumentation in order to document sociochemical norms and to examine their role in framing classroom discourse.
Rationale and research question
While social norms are often apparent from an examination of classroom participation patterns, empirical approaches for documenting epistemic criteria that influence how students use evidence in particular contexts are less well established. For example, Yackel et al. (2000) describe an emergent sociomathematical norm that is related to epistemic criteria used to evaluate mathematical arguments in an inquiry-oriented differential equations class (justifications were acceptable if they were grounded in an interpretation of rate of change), but the empirical steps taken to document this norm were not specified. In order to provide a more rigorous approach for documenting emergent norms, we adapted the methodological approach based on Toulmin's model of argumentation as used by Rasmussen and Stephan (2008) in a Process Oriented Guided Inquiry Learning (POGIL) physical chemistry classroom as a lens for classroom reasoning.In this study, we examined arguments produced in a POGIL physical chemistry classroom for evidence of classroom norms. In particular, we documented emergent norms related to discipline-specific criteria that pertain to justifying reasoning within chemistry contexts, which we refer to as sociochemical norms. The research question that is the focus of this paper is:
• What socially established epistemic criteria (sociochemical norms) enable and constrain classroom reasoning in a POGIL physical chemistry class?
Methods
Participants
The setting for this study was a physical chemistry class at a Midwestern comprehensive university. The class, one of two physical chemistry courses that were required for chemistry majors, met three times a week for 50 min each class period. Fifteen undergraduate students (ten females, five males) were enrolled in the course; all were pursuing degrees in chemistry at the time of the study. All participants had completed two semesters of general chemistry and two semesters of organic chemistry as prerequisites for the course; many had also taken other upper-division chemistry courses as well as one or more calculus courses. Since participants included both juniors and seniors, their coursework backgrounds beyond chemistry and math were varied. Some students in the class were familiar with the format of POGIL classes because they had taken other chemistry courses at the university that used the POGIL approach. However, since our initial focus of data analysis was on collective-level growth of ideas, we did not obtain detailed information about participants' coursework backgrounds and prior experiences during this semester of data collection.The course instructor, referred to by the pseudonym Dr Black, was experienced in using the POGIL instructional approach. She had taught physical chemistry using the POGIL approach for eight years and had also integrated POGIL materials into her general chemistry courses for several years.
As a large portion of each class period was spent with students engaged in small group discussions, our research team selected one of the four groups of students for observation during small group work. To make this selection, our research team observed each of the student groups during the first four weeks of the semester in the class in order to identify an information-rich case for our study. The focus group for this study was chosen because each of the four students routinely contributed to discussions, and they worked well together. Additionally, the membership of this group of students remained constant while other groups were re-organized by the instructor in order to improve small group dynamics. The group was comprised of four students, referred to by the pseudonyms Adam, Beth, Carrie, and Melissa. All of the focus group members were third-year chemistry majors. All had completed at least a general chemistry course prior to enrolment in Dr Black's physical chemistry course.
Classroom setting
A typical class period in this class began with Dr Black making announcements and opening the floor for questions about homework. Next, she would introduce the new content to be discussed during that class period, often providing an overview of the content covered in the POGIL workbook modules (called ChemActivities). ChemActivities in the POGIL workbook were organized around key themes in physical chemistry content, including enthalpy, and entropy, and equilibrium (Spencer et al., 2004). Critical Thinking Questions (CTQ's) from the workbooks prompted students to explain trends in data, make predictions about chemical and physical processes, and define terminology and symbolism as they worked together in small groups. Focus questions, designed to be answered without prior instruction of the new content, began each module and were frequently used as a way to introduce new topics.Students would typically work with their group members on designated questions from the POGIL workbook for an agreed upon amount of time (typically five to ten minutes) after the ChemActivity for the day had been introduced. After the allocated time had passed for group work, the instructor would lead a whole class discussion of CTQ's. At the instructor's request, the designated spokesperson from each group would share the group's reasoning to the CTQ's. The instructor would then guide discussions and provide mini-lectures as needed to clarify the class's understanding. During whole class discussions, Dr Black would lead discussion of the questions in order to clarify the class's reasoning about the CTQ's that had been previously discussed in small group work. Whole class discussions typically served as a space in which small groups would share reasoning to CTQ's and in which Dr Black could clarify reasoning related to these questions. This cycle was then repeated for the next set of CTQs.
Data collection
The focus of this study is on a five-week period that began the fourth week of the spring semester during which thermodynamics content was covered. Topics covered during this timeframe included work, heat, enthalpy, heat capacity, and entropy. Our interdisciplinary research team selected these thermodynamics topics as the focus of our research because this material involves a significant interplay between mathematical symbolism and abstract concepts. Data collection days and topics covered are summarized in Table 1.| Day | Date | Module | Content |
|---|---|---|---|
| 1 | 2/2/09 | T1 | Work |
| 2 | 2/4/09 | T2 | First law of thermodynamics |
| 3 | 2/6/09 | T3 | Enthalpy |
| 4 | 2/9/09 | T3A | Enthalpy |
| 5 | 2/11/09 | T4 | Heat Capacity |
| 6 | 2/13/09 | T4, T5 | Heat Capacity; Temperature Dependence of Enthalpy of Reaction |
| 7 | 2/16/09 | T5, T6 | Temperature Dependence of the Enthalpy of Reaction; Entropy |
| 8 | 2/18/09 | T7 | Enthalpy change as a function of temperature |
| 9 | 2/20/09 | T8 | Third law of thermodynamics |
| 10 | 2/23/09 | T8, T9 | Third law; Gibbs and Helmholtz energy |
| 11 | 2/25/09 | T9 | Gibbs and Helmholtz energy |
| 12 | 3/2/09 | T10 | Gibbs energy as a function of temperature and pressure |
The primary data for this study were video recordings of whole class and small group discussions. Video recordings of whole class discussions and focus group activity for the twelve class periods shown in Table 1 were transcribed verbatim. Observational notes of small group and whole class interactions were also collected and copies of student workbooks were obtained at the end of the semester.
Data analysis
The methodological approach used in this study is an extension of the process described by Rasmussen and Stephan (2008) for using Toulmin's model of argumentation as a way to document and analyse students' mathematical progress as it occurs in inquiry-oriented classrooms (Cole et al., 2012). The three-phases of this approach are summarized in Fig. 2. | ||
| Fig. 2 Summary of data analysis approach. | ||
Guided by the work of Rasmussen and Stephan (2008), we examined argumentation logs for shifts in function of particular pieces of information that would suggest a particular idea began to function as-if-shared by the classroom community (that is, the idea becomes normative). Specifically, we applied three criteria to identify normative ideas, the first two of which come from Rasmussen and Stephan (2008) and the third from Cole et al. (2012). First, we looked for instances where warrants or backings for a particular claim were initially present but then dropped off (Criterion 1). Second, we identified when certain parts of an argument shifted position in subsequent arguments, indicating knowledge consolidation (e.g., claim shifted to data) (Criterion 2). Third, we identified when a particular idea was used as justification for multiple claims across different class periods (Table 2).
| Criterion 1 | When warrants or backings are initially present but then drop off |
| Criterion 2 | When an idea shifts position in subsequent arguments indicating knowledge consolidation |
| Criterion 3 | When particular ideas are repeatedly used as justification for different claims on different days |
These three criteria were used in a previous paper to identify classroom chemistry practices (Cole et al., 2012). This analysis and resulting argumentation logs served as a starting point for the sociochemical norm analysis.
After identifying potential themes, we examined the original video and transcript data and made conjectures about classroom norms underlying trends in classroom reasoning. An important feature of this phase of our analysis was the use of negative cases (those that appear to contradict an emerging theme) in order to confirm and refine emerging categories of classroom norms. In our data, we found that rebuttals and counterexamples often signalled a negative case in which evidence was used in a non-normative fashion. In such instances, we observed the class's response to apparent breaches in normative activity, which could either confirm or refute our interpretation of classroom norms underlying argumentation activity.
Reliability
In a continuous data set, such as transcripts of classroom discourse, it is important to drive towards a reliable application of a coding scheme and agreement across researchers. To this end, our research team met at the beginning of the project to collaboratively code a portion of the whole class transcripts using Toulmin's model. As a research team we coded a portion of the whole class discussion data in collaboration so that a consistent use of our coding scheme was established. Later, members of the research team coded transcripts individually, and we compared our results at periodic project meetings until a complete agreement was reached on all twelve transcripts. Small group work was initially coded by the entire research team and as the project progressed a smaller subset of researchers collaborated to complete coding of the small group work. As with the whole class discussions, coding was discussed until agreement was reached.Findings
Based on our analysis using Toulmin's model of argumentation, we noted several themes in the characteristics of data, warrants, backings, and rebuttals across classroom arguments. One theme, which emerged from our use of Criterion 3, was that the class repeatedly used justifications related to particulate-level ideas in order to justify reasoning about chemical and physical properties. Upon examination of the classroom context of these data, we considered the patterns in which these particulate-level justifications were used to reflect a tacit criterion related to what counts as acceptable types of justifications, that is, a sociochemical norm. In this section we illustrate how this sociochemical norm was established in Dr Black's physical chemistry class and how it shaped classroom reasoning about chemical and physical properties.Sociochemical norm: justifying reasoning using particulate-level evidence
In analysing arguments in both small group and whole class discussion, we observed that across the data set Dr Black's physical chemistry class routinely used information about the motion and spacing of particles or information about molecular structure as data, warrants, and backings for claims about chemical and physical properties. This seemed particularly interesting given the well-documented difficulty students have in relating particulate descriptions of matter to chemical and physical properties and evidence in previous studies that students tended to resist using particulate-level explanations for chemical phenomena (e.g., Abraham et al., 1994; Cooper et al., 2010).In the discussion that follows, we use the term ‘particulate’ to refer to characterizations based on quantities of particles and their interactions as well as molecular models related to the bonds and structure within an individual molecule. Information about the motion and spacing of a collection of particles was the most frequently used type of particulate type of justification for claims about chemical and physical properties. Less frequently, descriptions of molecular structure were also referenced.
Even though there was significant variation in the types of particulate warrants and backings that were considered appropriate across the five-week data collection period, the occurrence of rebuttals and counterarguments in instances where particulate-level evidence was not appropriately used suggested that there was an emerging expectation with respect to how these ideas could be used to support claims. The ways in which particulate descriptions were used by the class suggested that justifying reasoning using particulate-level evidence had become normative for the class. In the following sections, we illustrate this norm and the classroom interactions through which it was enacted.
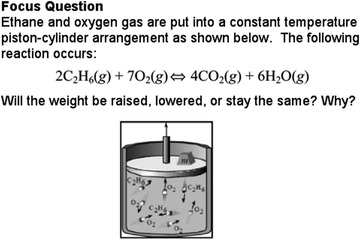 | ||
| Fig. 3 Focus question from unit on enthalpy from POGIL workbook. Reprinted from Physical chemistry: Guided inquiry thermodynamics (p. 71), by Spencer et al., 2004, Boston, MA: Houghton Mifflin Company. Reprinted with permission. | ||
As the group negotiated a response to this question, several types of evidence were proposed as justification for the claim that the piston would be raised. Adam's initial justification for this claim referenced an analogy to everyday experience, combustion in a gasoline engine. This reasoning is summarized by the following argument (note that italics in argumentation logs denote paraphrased dialogue while direct quotations are not italicized).
Argument 1a, Small Group Discussion from 2/9/09
Claim: Weight would be raised (Melissa)
Data: It's like a car (Adam)
Rebuttal: I would like to know why it's raised, cause we're using the exact same amount. (Carrie)
Here, Carrie immediately challenged the relevance of the data used in this argument, as illustrated by the following transcript excerpt.
Carrie: I would like to know why it's raised, cause we're using the exact same amount…
Adam: I, I think, think, yeah but there's moles of things, there's 10.
Carrie: There's still the same number of hydrogen's, still the same number of oxygen's, which is how you determine, like, the molar mass that it would take?
Adam: Right, but there's more molecules. But, ok. This is a big one, so there's like two or three big ones in this one, and there's smaller ones that are like 10 or 15 smaller ones.
As illustrated by this transcript excerpt, small group discussions within the focus group tended to be highly interactive, with each member of the group contributing to discussions. Often, group members would restate and refine one another's ideas and thus the co-constructed collective arguments often involved multiple iterations of an argument as evidence was refined.
In response to Carrie's rebuttal, Adam provided an alternative argument that used the molar ratio (ten moles of product molecules for nine moles of reactant) as data instead of an analogy (Argument 1b).
Argument 1b, Small Group Discussion from 2/9/09
Claim: Weight would be raised (Melissa)
Data: Yeah, but there's more things, there's 10 [gestures towards balanced chemical reaction as if to referring to the relative number of moles of products and reactants] (Adam)
Rebuttal: There's still the same number of hydrogen's, still the same number of oxygen's, which is how you determine [weight] (Carrie)
Again, Carrie requested further elaboration. As group members requested elaboration and provided rebuttals and counterclaims, the group refined the data used to support the claim that the piston would be raised (Arguments 1c and 1d).
Argument 1c, Small Group Discussion from 2/9/09
Claim: Weight would be raised (Melissa)
Data: A greater number of smaller molecules are produced as products (Adam)
Warrant: Smaller molecules move faster and produce more pressure (Adam)
Argument 1d, Small Group Discussion from 2/9/09
Claim: Weight will be raised (Beth)
Data: The products are a greater number of smaller molecules (Beth)
Warrant: Smaller molecules move faster (Beth)
Backing: Moving faster means they would collide more with the container, which means more pressure (Beth)
In Argument 1d, Beth reiterated the group's claim and data, but elaborated on the warrant, providing a backing for the emerging argument. By restating the group's previous argument, she checked her interpretation of the group's reasoning and also affirmed that their group had reached a consensus. An interesting feature of this particular example was that Carrie revealed later in the discussion that the source of her confusion was that she interpreted the “weight” referenced by the group as the molar masses of the products compared to the reactants rather than a physical weight representing the external pressure on the piston. Carrie's assumption that “weight” referenced a quality at the particulate-level of the products further evidences the importance of the sociochemical norm of justifying reasoning using particulate-level evidence. In this instance, students' interactions served not only to refine their use of particulate-level information as evidence, but also to refine their use of terminology by requiring that all group members held common interpretations.
In this sequence of arguments, the group presented three different types of evidence as justification for the claim that the weight pictured in the diagram would be raised. Initially, Adam suggested analogical evidence derived from his everyday understanding of how a car engine worked (data, Argument 1a); Next, Adam and Carrie's interpretation of the balanced chemical equation served as data (data, Argument 1b); In the end, a particulate-level description of the process served as acceptable evidence (warrant, Arguments 1c and 1d). Adam's initial attempt at justification using evidence from everyday experience was abandoned as the group negotiated an argument that was supported by a particulate-level description of the chemical process. This type of reasoning approximated previous justifications that had been used in whole class discussions. Thus, we believe the group's justification to be shaped by a criterion for what counts as an acceptable justification, namely that justifications should appeal to particulate-level descriptions of matter.
Sociochemical norm in whole class discussion
During whole class discussions in this POGIL physical chemistry class, Dr Black typically initiated discussion of the POGIL workbook questions and elicited responses from students. The majority of arguments in whole class discussion were thus co-constructed between students and the instructor. In terms of Toulmin's model of argumentation, the instructor's contributions most often served as warrants and backings for the class's co-constructed claims about chemical and physical properties. The following argument illustrates how a particulate-level warrant was used to justify a claim about enthalpy of reaction.Argument 2a, Whole Class Discussion from 2/18/09
Claim: Enthalpy of reaction is positive for the melting of ice (Dr Black, restates claim provided by workbook)
Data: Because it's going from a solid to a liquid (Zane)
Warrant: Going from a solid to a liquid requires heat because it [the solid] breaks down (Zane)
Revised Warrant: We put energy in to go from solid to the liquid so we give the molecules enough energy to move around (Dr Black, rephrases student warrant)
Here, students provided the initial data and warrant (in keeping with the normative pattern of participation established in this class), and the instructor elaborated on students' responses to refine and extend the class's reasoning.
In another instance, the class used particulate evidence in order to reason about the standard state entropies of solids, liquids, and gases. At the beginning of the class period, Dr Black defined entropy as the number of ways energy or particles could be distributed within a system. In the whole class discussion that followed, she prompted the students to consider which would have greater standard state entropy, large molecules or small molecules. The arguments from this discussion are as follows:
Argument 3a, Whole Class Discussion from 2/16/09
Claim: Bigger molecules have more entropy (Dr Black)
Data: There are more electrons in bigger molecules (Dr Black)
Warrant: There are more ways to distribute them (Dr Black)
Argument 3b, Whole Class Discussion from 2/16/09
Claim: Gas has the most entropy (Multiple students)
Data: It has the least interactions (Luke/Helen)
Warrant: I don't really have any restrictions on where I put the gas molecules (Dr Black)
Backing: There are a lot of ways to distribute the particles (Dr Black)
Argument 3c, Whole Class Discussion from 2/16/09
Claim: Solids have the least amount of entropy (Multiple students)
Data: Can't change it; atoms in a fixed position (Jane)
Warrant: Particles in solids have fixed positions (Dr Black)
Argument 3d, Whole Class Discussion from 2/16/09
Claim: The standard state entropies of liquids are in between those of gases and solids (Dr Black)
Data: They're moving around a little bit, but not as far as in gases (Marie)
Warrant: They can't just go moving off, we still have forces and interactions. (Dr Black)
Again in these arguments, descriptions of how particles could be distributed in solid, liquid, and gas phase substances were used to justify why solids, liquids, and gases would have different standard state entropies. In Arguments 3c and 3d, the instructor expanded on student-contributed claims to provide warrants, moves that served both to validate students' use of particulate-level evidence and to further articulate the relationship between the data and the claims.
A third example in which the class used particulate-level ideas as data occurred several class periods later during a discussion of the Third Law of Thermodynamics. During this class period, the class discussed a CTQ in which they were asked to explain why all materials must be solids at absolute zero. The arguments for this exchange follow.
Argument 4a, Whole Class Discussion from 2/20/09
Claim: All materials must be solid at absolute zero (Text)
Data: There is no motion (Andrea)
Alternative Data: The way its compact (Andrea)
Rebuttal: Ok you're sure dancing around it (Dr Black)
Argument 4b, Whole Class Discussion from 2/20/09
Claim: All materials must be solid at absolute zero (Text)
Data: There's no room to move (Andrea)
Rebuttal: It doesn't have to do with space available (Dr Black)
Argument 4c, Whole Class Discussion from 2/20/09
Claim: All materials must be solid at absolute zero (Text)
Data: The particles move in a crystal structure (Tom)
Rebuttal: No they don't have to, we can have amorphous solids (Dr Black)
Argument 4c, Whole Class Discussion from 2/20/09
Claim: All materials must be solid at absolute zero (Text)
Data: You don't have any net translational motion where they're shifting positions (Dr Black)
Here, students made several attempts to justify why a material must be a solid at absolute zero by appealing to descriptions of the motion and spacing of solid particles. Their attempts ranged from describing no motion in a solid at all (Andrea, Argument 4a), a lack of room for movement to occur (Andrea, Argument 4b), and movement within a crystal structure arrangements of solids (Tom, Argument 4c). Ultimately, Dr Black contributed the evidence that in a solid, where particles are in fixed positions relative to one another, there is no net translational motion of solid particles after which the class further discussed the relationship of translational motion to temperature.
In the previous arguments, ideas about the motion and spacing of solid, liquid, and gas particles were used as evidence. Similarly, particulate-level ideas were used throughout the data collection period to justify chemical and physical properties across different content, including topics of enthalpy, entropy, and the Third Law of Thermodynamics. Such persistent use of similar evidence suggests that particulate-level explanations had become a normative type of explanation for justifying reasoning about chemical and physical properties.
However, this episode shows that constructing appropriate arguments using particulate-level ideas was not without difficulty for the students in Dr Black's class. As in the previous examples, students at times struggled to relate particulate ideas to more abstract constructs such as entropy or enthalpy and did not always use particulate-level data appropriately. These examples highlight an important role of the instructor during whole class discussion, which was to help the class to qualify the use of particulate-level information and to select the most appropriate type of particulate evidence for a particular claim. In such instances, the role of the instructor was to reframe student-provided evidence into more discipline-appropriate forms in order to scaffold classroom reasoning.
Similarly, sociochemical norms in Dr Black's physical chemistry class evolved over the data collection period as the class encountered new content and re-negotiated classroom norms through their interactions. Early in the data collection period, data and warrants related to the particulate nature of matter most often involved descriptions of the motion and spacing of particles. Later in the semester, however, a second relevant type of particulate-level data became more widely used, which involved descriptions of the structure of individual molecules. This was likely because the type of particulate evidence that was best suited for making a claim about entropy was not necessarily the type of evidence that would be most appropriate for reasoning about another topic like heat capacity. Due to differences in the content discussed, sociochemical norms related to reasoning using particulate-level descriptions of matter broadened to include new ways of using particulate explanations for new content topics.
However, across the content, there remained an expectation that particulate-level explanations be used to make claims about chemical and physical properties. For instance, during the following whole class discussion, the focus group shared their reasoning for a critical thinking question related to heat capacities of gas samples (Fig. 4).
 | ||
| Fig. 4 Critical thinking question from unit on heat capacity in POGIL workbook. Reprinted from Physical chemistry: Guided inquiry thermodynamics (p. 79), by Spencer et al., 2004, Boston, MA: Houghton Mifflin Company. Reproduced with permission. | ||
In discussing this question, all student groups had constructed arguments that were justified using particulate-level descriptions of the motion and spacing of gas particles, even though students had likely discussed how molecular structure relates to possible energy modes in previous coursework (especially those students who had already taken the physical chemistry course focused on quantum mechanics). The focus group's argument as reported to the whole class follows.
Argument 5a, Whole Class Discussion from 2/11/09
Claim: Neon and nitrogen will have two different temperatures even if the same amount of energy is added (Beth)
Data: Nitrogen (N2) is bigger (Beth)
Warrant: Bigger molecules take more heat to move (Beth)
Rebuttal: Um, that's, your statement is true, I don't think it will have a big impact on, the, uh, temperature. (Dr Black)
Since similar types of particulate-level evidence had been used when reasoning about different concepts in previous class periods, it seems reasonable that the class understood that this type of particulate justification would be appropriate in this context.
However, Dr Black's rebuttal to Beth's data seemed to signify a breach of an existing norm related to what counts as acceptable use of evidence in this context, in particular, what would count as an acceptable particulate-level justification. As the discussion continued, Dr Black questioned the class in order to generate an alternative argument.
Dr Black: If all of it doesn't become kinetic energy then some of it becomes something else. So if it's more massive then does that make it have something else that, will nitrogen have something that neon doesn't just because it's bigger? So if I was comparing argon and neon, would there be a difference?
Andrea: No.
Dr Black: No, so it's not just size, cause those are different sizes. What is it that nitrogen has that neon doesn't?
Helen: It has rotation.
Dr Black: It has rotations. Cause here when we were talking about kinetic energy, what are, what types of motion we're talking about?
Helen: Translation?
Dr Black: We're talking about translational motion. So when I look at N2, it has a bond. So it can rotate, what else can it do?
Multiple: Vibrate.
Dr Black: And it can vibrate. So I look at N2, it can take some of that q [heat energy] and put it into rotational motion and vibrational motion, so this is where my group over here is like well, we get more energy states. Yes, cause now I can occupy higher vibrational states and higher rotational states.
In this whole class exchange, Dr Black directed students to consider descriptions of molecular structure in the case of diatomic nitrogen as evidence for the claim that nitrogen would have a lower final temperature. Later in the discussion, she continued her line of questioning in order to compare the structure of neon with that of nitrogen.
Dr Black: Why doesn't neon have any rotational energy?
Craig: The bond.
Helen: Because it looks the same however it moves?
Dr Black: I have to have an axis of rotation to be able to tell that there's a difference that it's moved.
Again, Dr Black elaborated on Helen's contribution (“Because it looks the same however it moves?”) in order to shift the discussion towards more relevant features of the particulate-level structures of neon and nitrogen (e.g. “I have to have an axis of rotation to be able to tell that there's a difference that it's moved”). Through her interaction with the class, the instructor modelled a more appropriate use of particulate-level information as evidence for this claim as shown in Arguments 5b and 5c.
Argument 5b, Whole Class Discussion from 2/11/09
Claim: Nitrogen can rotate and neon can't rotate (Helen)
Data: Nitrogen has a bond (Dr Black)
Argument 5c, Whole Class Discussion from 2/11/09
Claim: Nitrogen has a lower temperature than neon (Dr Black)
Data: It can vibrate and rotate and neon can't (Students)
Warrant: Some of the heat energy can be put into rotational and vibrational motion (Dr Black)
Backing: Only the translational energy is kinetic energy (Dr Black)
In this example, Dr Black did not directly indicate whether students' contributions were correct or incorrect. Instead, she rephrased and refined student contributions, which indicated to the class that the response was appropriate. Such contributions served to shape ideas of what counts as acceptable particulate-level explanations in different contexts.
Relationship of whole class reasoning to small group reasoning
As illustrated by the previous examples, the instructor played a significantly more prominent role in orchestrating whole class compared to small group discussions. In small group work, however, there was evidence that students took on the role of providing evaluative argumentation moves such as rebuttals and counterarguments. In contrast with whole class arguments, small group arguments included a greater number of student-contributed counterclaims and rebuttals. In some contexts, these rebuttals occurred as students pressed one another to reframe evidence in ways that more closely approximated the normative ways of reasoning that were present in whole class discussions.For instance, during a unit on entropy, the class used particulate evidence in order to reason about the standard state entropies of solids, liquids, and gases. During the whole class introduction of a unit on entropy, the instructor defined entropy as the number of ways energy or particles could be distributed within a system. As previously shown in Arguments 3a through 3d, descriptions of how particles could be distributed in solid, liquid, and gas phase were used to justify why solids, liquids, and gases would have different standard state entropies during a whole class discussion.
Following this whole class discussion in which Arguments 3a–3d took place, the focus group worked on a CTQ related to the spontaneity of various processes (Fig. 5). The small group's initial reasoning for this relied on evidence related to energy changes for the process, and was not related to the motion and spacing of particles.
 | ||
| Fig. 5 Critical thinking question from unit on entropy in POGIL workbook. Reprinted from Physical chemistry: Guided inquiry thermodynamics (p. 94), by Spencer et al., 2004, Boston, MA: Houghton Mifflin Company. Reprinted with permission. | ||
Argument 6a, Small Group Work from 2/16/09
Claim: If (Stot)final > (Stot) initial for a process, the process would be considered spontaneous (Beth)
Data: ΔStot > 0 (Beth)
Warrant: If your final is bigger than your initial that means your ending thing is more energy than your starting (Beth)
Backing: Spontaneous means energy is not necessary for a process to occur. (Carrie)
Though the relationship to energy was not explicitly rebutted through the course of the exchange, the group's justification in terms of energy was replaced with an explanation of entropy in terms of the organization of particles, a definition that was also used in subsequent class periods.
Argument 6b, Small Group Work from 2/16/09
Claim: Entropy of the universe increases for a spontaneous process (Beth)
Data: Particles become more spread out in the final state than initial state (Adam)
Warrant: Entropy is the organization/distribution of particles (Adam)
Backing: Ok, oh yeah cause we're trying to get to bigger S. We always want bigger S. Right bigger S. (Adam)
This justification that was grounded in a particulate-level description of the organization of the system more closely approximated that used in the preceding whole class discussions. The group's reasoning was not entirely correct in that the entropy of the system and entropy of the universe were conflated. However, the small group's adoption of a particulate-level justification similar to that previously used in whole class discussions suggests that the group's reasoning was compatible with the expectation that reasoning should appeal to particulate-level descriptions, and the correct aspect of the particulate nature of matter was used.
Prevalence of particulate-level evidence in reasoning
It is noteworthy that the frequencies with which particulate explanations were used in whole class discussion did not remain constant across the data set. Instead, we observed a cyclic pattern in the frequency with which particulate explanations were used. Often, particulate-level evidence was often initially present when a concept was introduced, but then dropped off as the class became more familiar with the concept. For example, data and warrants related to interpretations of mathematical expressions would take the place of particulate-level evidence as a concept was developed. However, if the class moved on to a new topic (such as the introduction of entropy following a module on heat capacity), explicit use of particulate ideas as evidence would often reappear. This is not to say that reasoning using particulate-level ideas ceased to be a normative type of reasoning for the class; Rather, particulate ideas may have functioned as implicit components of reasoning such as backings that were no longer articulated by the class (Rasmussen and Stephan, 2008).The cyclic pattern of particulate-level evidence use may also be related to the structure of the POGIL workbooks. The workbook used by the class Spencer et al., (2004)'s Physical Chemistry: Guided Inquiry Thermodynamics, was consistently structured such that as new concepts such as entropy, or heat capacity were introduced, the workbooks modules would initially include a greater number of questions that asked for explanations or predictions related to various scenarios. For example, a module on enthalpy in the POGIL workbook began with the diagram presented earlier in Fig. 3. This question, which was a Focus Question, presented a diagram showing a piston-cylinder and a chemical reaction for the combustion of ethane. Students were to predict what would happen to the cylinder if the reaction were carried out. Questions that focused on qualitative explanations were often used early after the introduction of new content and were designed to elicit prior knowledge rather than applications of new material.
However, during the latter portions of POGIL workbook modules, there was often a shift towards the use of mathematical expressions as data and warrants rather than particulate-level ideas. This may be related to the fact that CTQ's in the latter half of the POGIL ChemActivities used by the class would require that students perform derivations or apply previously learned equations or formulae. For example, a CTQ that occurred later in the same module as the question in Fig. 4 asked students to “Recall how dU is related to dq and dw. Use your answer to CTQ 3 to provide an expression showing the relationship between dH and dq for a constant pressure process (Spencer et al., 2004, p. 63). The nature of the Focus Question in Fig. 4 compared to the CTQ described above, which asked for a derivation, required that students use different types of reasoning in their response to the questions. While the first example is designed to elicit intuitive understandings and prior knowledge, the second explicitly calls for an application of mathematical relationships. Correspondingly, in our analysis of classroom argumentation in Dr Black's physical chemistry class, we observed a shift towards interpretations of mathematical expressions as justifications as opposed to particulate-level ideas. Thus, multiple types of normative justifications may be present within a classroom and their use is influenced both by the instructor and the curriculum. Further discussion of other sociochemical norms that were present in Dr Black's physical chemistry class, and the relation of these norms to one another will be presented in a later manuscript.
Summary of findings
In response to our research question posed above, we described the sociochemical norm that one type of acceptable explanation relating to concepts such as heat energy, enthalpy, heat capacity, and entropy is grounded in particulate-level descriptions of chemical systems. The recurrent use of particulate-level descriptions as data and warrants provided empirical evidence for the presence of this sociochemical norm; furthermore, rebuttals and counterarguments in response to breaches of the emerging norm served to confirm that particulate-level justifications served as a normative type of reasoning in this classroom. In small group work, particulate-level ideas were used as justifications in preference of evidence derived from analogy or everyday experience. Ideas of how particulate-level explanations were to be used and under what conditions they were appropriate were refined in whole class discussion as the instructor scaffolded classroom reasoning.It is important to note, however, that justifications that appealed to particulate-level descriptions of matter were not the only type of evidence that was considered acceptable in this classroom. Interpretations of mathematical expressions and information related to energy transfer were also among the normative types of explanations used to reason about chemical and physical properties. However, a key role of the instructor remained to help students construct arguments using evidence appropriate to the context.
Limitations
The study provides a view of how social and sociochemical norms were enacted in only one physical chemistry classroom over the course of a five-week period. It has been noted that the ways in which students engage in reasoning and argumentative practices are highly dependent on classroom culture and the ways in which teachers facilitate classroom interactions (Berland, 2011; Berland and Reiser, 2011). Thus, the findings reported here represent a case study of classroom norms, rather than a generalizable view of classroom norms across chemistry contexts. In our future work, we plan to extend this approach to analysing classroom norms to include data from multiple classrooms and longer time frames of data collection in order to provide a more complete description of how classroom norms are established over the course of the semester.Conclusions and implications
A significant body of literature exists in chemistry education research that has examined individual students' conceptual understandings of the particulate nature of matter. Numerous misconceptions related to the particulate nature of matter have been well documented in science education literature across age levels (Abraham et al., 1994; Cooper et al., 2010; Kelly and Jones, 2007; Kelly and Jones, 2008; Novick and Nussbaum, 1981).While individual understandings of particulate-level ideas certainly comprise one aspect of student understanding, we view the ways in which students relate particulate ideas to various thermodynamic topics as highly dependent on socially negotiated classroom criteria as to what counts as appropriate use of evidence. Because the ways in which students use particulate-level evidence to construct an understanding of more advanced chemistry concepts is framed by participation in discipline-specific norms, we contend that the largely tacit, social and sociochemical norms comprise critical aspects of student learning of chemistry that merit further exploration. Our work suggests that beyond having a conceptual understanding of the particulate nature of matter, students in chemistry must become able to use particulate-level evidence to reason about chemical and physical properties. That is, they must be able to construct arguments using particulate-level ideas and representations.
While there are many ways to characterize individual learning, far fewer tools exist to help understand what goes on at the classroom level. One of the most important findings of the work described herein is the explication and dissemination of tools chemistry education researchers can use to investigate classroom level dynamics. Social factors play a non-trivial role in framing the classroom learning environment and their impact on student learning could certainly be more fully explored. Additionally, more work is needed in order to coordinate individual and social views of learning.
Work on sociomathematical norms has illustrated ways in which particular classroom activities seemed to promote the emergence of sociomathematical norms (McClain and Cobb, 2001; Rasmussen et al., 2003). Identifying and relating norms to the classroom context in which they occur may be able to promote the emergence of particular norms aligned with discipline-specific content learning goals. For instance, requesting that students share reasoning and explain how graphical or symbolic representations relate to particulate-level reasoning may be one way to facilitate a norm that evidence be grounded in particulate-level descriptions of chemical or physical processes. Furthermore, while the negotiation of the sociochemical norm that explanations should be grounded in particulate-level descriptions was largely implicit in the classroom in this study, a more explicit negotiation of such norms may be beneficial to students.
In conclusion, describing the ways in which chemical ideas are used in a dynamic setting represents a new direction for chemistry education research as this work examines one theme for reasoning that emerges between and amongst the students and the instructor in a naturalistic setting. The construct of sociochemical norms is a pragmatic lens for exploring students' reasoning in collaborative settings.
Acknowledgements
This work is supported by the National Science Foundation under grants #0816792, #0817467, #0816948. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We also wish to thank the physical chemistry students who took part in this study.References
- Abraham, M. R., Williamson, V. M., and Westbrook, S. L. (1994). A cross-age study of the understanding of five chemistry concepts, J. Res. Sci. Teach., 31, 147–165.
- Asterhan, C. S. C., and Schwarz, B. B. (2007). The effects of monological and dialogical argumentation on concept learning in evolutionary theory, J. Educ. Psychol., 99, 626–639.
- Berland, L. K. (2011). Explaining variation in how classroom communities adapt the practice of scientific argumentation, J. Learn. Sci., 20, 625–664.
- Berland, L. K., and Lee, V. R. (2012). In pursuit of consensus: Disagreement and legitimization during small-group argumentation, Int. J. Sci. Educ., 34, 1857–1882.
- Berland, L. K., and Reiser, B. H. (2011). Classroom communities' adaptations of the practice of scientific argumentation, Sci. Educ., 95, 191–216.
- Bowers, J., and Nickerson, S. (2001). Identifying cyclic patterns of interaction to study individual and collective learning, Math. Think. Learn., 3, 1–28.
- Bricker, L. A., and Bell, P. (2008). Conceptualizations of argument from science studies and their implications for the practices of science education, Sci. Educ., 92, 473–498.
- Cobb, P., Stephan, M., McClain, K., and Gravemeijer, K. (2001). Participating in classroom mathematical practices, J. Learn. Sci., 10, 113–163.
- Cobb, P., and Yackel, E. (1996). Constructivist, emergent, and sociocultural perspectives in the context of developmental research, Educ. Psychol., 31, 175–190.
- Cole, R., Becker, N., Towns, M. H., Sweeny, G., Wawro, M., and Rasmussen, C. (2012). Adapting a methodology from mathematics education research to chemistry education research: Documenting collective activity, Int. J. Sci. Math. Educ., 10, 193–211.
- Cooper, M., Grove, N., Underwood, S. M., and Klymkowsky, M. W. (2010). Lost in Lewis structures: An investigation of student difficulties in developing representational competence, J. Chem. Educ., 87, 869–874.
- Corbin, J., and Strauss, A. (2008). Basics of qualitative research: Techniques and procedures for developing grounded theory (3rd edn). Thousand Oaks, CA: Sage Publications.
- Creswell, J. (2007). Qualitative inquiry and research design: Choosing among five approaches (2nd edn). Thousand Oaks, CA: Sage Publications.
- Driver, R., Newton, P., and Osborne, J. (2000). Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84, 289–312.
- Duschl, R. A., and Osborne, J. (2002). Supporting and promoting argumentation discourse in science education, Stud. Sci. Educ., 38, 39–72.
- Eberlein, G., Kampmeier, J., Minderhout, V., Moog, R. S., Platt, T., Varma-Nelson, P., and White, H. B. (2008). Pedagogies of engagement in science: A comparison of PBL, POGIL, and PLTL, Biochem. Mol. Biol. Educ., 36(4), 262–273.
- Erduran, S. (2007). Methodological foundations in the study of argumentation in science classrooms. In S. Erduran and M. P. Jimenez-Aleixandre (ed.), Argumentation in science education: Recent developments and future directions (pp. 47–69). Dordreht: Springer Academic Publishers.
- Erduran, S., Simon, S., and Osborne, J. (2004). TAPping into argumentation: Developments in the application of Toulmin's Argument Pattern for studying science discourse, Sci. Educ., 88, 915–933.
- Farrell, J. J., Moog, R. S., and Spencer, J. N. (1999). A guided inquiry chemistry course, J. Chem. Educ., 76, 570–574.
- Ford, M. J., and Forman, E. (2006). Redefining disciplinary learning in classroom contexts, Rev. Educ. Res., 30, 1–32.
- Forman, E., Larreamendy-Joerns, J., Stein, M. K., and Brown, C. A. (1998). “You're going to want to find out which and prove it”: Collective argumentation in a mathematics classroom, Learn. Instr., 8, 527–548.
- Hanson, D. M., and Apple, D. K. (2004). vol. IV: Process-oriented guided inquiry learning–assessment, What works, what matters, what lasts. Retrieved from http://www.pkal.org/open.cfm?d_id=363.
- Hanson, D. M., and Wolfskill, T. (1998). Improving the teaching/learning process in general chemistry, J. Chem. Educ., 75, 143–147.
- Hanson, D. M., and Wolfskill, T. (2000). Process workshops: A new model for instruction, J. Chem. Educ., 77, 120–130.
- Inglis, M., Mejia-Ramos, J. P., and Simpson, A. (2007). Modelling mathematical argumentation: The importance of qualification, Educ. Stud. Math., 66, 3–21.
- Jiménez-Aleixandre, M. P., and Erduran, S. (2007). Argumentation in science education: An overview. in S. Erduran and M. P. Jimenez-Aleixandre (ed.), Argumentation in science education: Recent developments and future directions. Dordreht: Springer Academic Publishers.
- Kelly, R. M., and Jones, L. L. (2007). Exploring how different features of animations of sodium chloride dissolution affect students' explanations, J. Sci. Educ. Technol., 16, 413–429.
- Kelly, R. M., and Jones, L. L. (2008). Investivating students' ability to transfer ideas learned from molecular animations of the dissolution process, J. Chem. Educ., 85, 303–309.
- Krummheuer, G. (1995). The ethnography of argumentation. In P. Cobb and H. Bauersfeld (ed.), The emergence of mathematical meaning: Interactions in classroom cultures (pp. 229–269). Hillsdale, NJ: Erlbaum.
- Krummheuer, G. (1998). Formats of argumentation in the mathematics classroom. in H. Steinbring, M. G. Bartolini Bussi and A. Sierpinska (ed.), Language and communication in the mathematics classroom (pp. 223–234). Reston, VA: National Council of Teachers of Mathematics.
- Kuhn, D. (2010). Teaching and learning science as argument, Sci. Educ., 94, 810–824.
- Kuhn, D., and Udell, W. (2003). The development of argumentation skills, Child Dev., 74, 1245–1260.
- Lawson, A. E. (2009). Basic inferences of scientific reasoning, argumentation and discovery, Sci. Educ., 94, 336–364.
- Lemke, J. L. (1990). Talking science: Language, learning, and values. Norwood, NJ: Ablex.
- Lewis, S. E., and Lewis, J. E. (2005). Departing from lectures: An evaluation of a peer-led guided inquiry alternative, J. Chem. Educ., 82(1), 135–139.
- McClain, K., and Cobb, P. (2001). An analysis of development of sociomathematical norms in one first-grade classroom, J. Res. Math. Educ., 32, 239–266.
- Moog, R. S. (2006). The POGIL Project: NSF-CCLI Phase 3 DUE-0618746.
- Moog, R. S., and Spencer, J. N. (2008). POGIL: An overview. In R. S. Moog and J. N. Spencer (ed.), Process Oriented Guided Inquiry Learning (POGIL), Washington, DC: American Chemical Society.
- Novick, S., and Nussbaum, J. (1981). Pupils' understanding of the particulate nature of matter: A cross-age study, Sci. Educ., 65, 187–196.
- Osborne, J. (2010). Arguing to learn in science: The role of collaborative, critical discourse, Science, 328, 463–466.
- Osborne, J., and Patterson, A. (2011). Scientific argument and explanation: A necessary distinction?, Sci. Educ., 95, 627–638.
- Process Oriented Guided Inquiry Learning. (2012). Retrieved May 2, 2012, from http://www.pogil.org.
- Rasmussen, C., and Stephan, M. (2008). A methodology for documenting collective activity. in A. E. Kelly, R. A. Lesh and J. Y. Baek (ed.), Handbook of innovative design research in science, technology, engineering, mathematics (STEM) education (pp. 195–215). New York: Taylor and Francis.
- Rasmussen, C., Yackel, E., and King, K. (2003). Social and sociomathematical norms in the mathematics classroom. in H. Schoen and R. Charles (ed.), Teaching mathematics through problem solving: Grades 6–12 (pp. 143–154). Reston, VA: National Council of Teachers of Mathematics.
- Sampson, V. D., & Clark, D. B. (2009). The impact of collaboration on the outcomes of scientific argumentation. Science Education, 93, 448-484.
- Sandoval, W. A., and Millwood, K. A. (2008). What can argumentation tell us about epistemology. in S. Erduran and M. P. Jimenez-Aleixandre (ed.), Argumentation in science education: Recent developments and future directions (pp. 71–88). Dordreht: Springer Academic Publishers.
- Spencer, J. N. (1999). New directions in teaching chemistry, J. Chem. Educ., 76, 566–569.
- Spencer, J. N., Moog, R. S., Creegan, F. J., Hanson, D. M., Wolfskill, D. M., Straumanis, A., and Bunce, D. M. (2003). Process Oriented Guided Inquiry Learning: NSF (DUE-0231120).
- Spencer, J. N., Moog, R. S., and Farrell, J. J. (2004), Physical chemistry: Guided inquiry thermodynamics. Boston: Houghton Mifflin Company.
- Stephan, M., and Rasmussen, C. (2002). Classroom mathematical practices in differential equations, J. Math. Behav., 21, 459–490.
- Toulmin, S. (1958), The uses of argument. Cambridge: Cambridge University Press.
- Vygotsky, L. S. (1978), Mind in society: The development of higher psychological processes. Cambridge, MA: Harvard University Press.
- Weber, K., Maher, C., Powell, A., and Lee, H. S. (2008). Learning opportunities from group discussions: Warrants become the objects of debate, Educ. Stud. Math., 68, 247–261.
- Yackel, E. (2001). Explanation, justification, and argumentation in mathematics classrooms. In M. van den Heuvel-Panhuizen (Ed.), Proceedings of the 25th International Conference on the Psychology of Mathematics Education, Vol. 1 (Vol. 1, pp. 9–23). Utrecht, Holland: IGPME.
- Yackel, E. (2002). What we can learn from analyzing the teacher's role in collective argumentation, J. Math. Behav., 21, 423–440.
- Yackel, E., and Cobb, P. (1996). Sociomathematical norms, argumentation, and autonomy in mathematics, J. Res. Math. Educ., 27, 458–477.
- Yackel, E., Rasmussen, C., and King, K. (2000). Social and sociomathematical norms in an advanced undergraduate mathematics course, J. Math. Behav., 19, 275–287.
- Zohar, A., and Nemet, F. (2002). Fostering students' knowledge and argumentation skills through dilemmas in human genetics, J. Res. Sci. Teach., 39, 35–62.
Footnote |
| † On 1 January 2013, this authors affiliation will change to Michigan State University, Department of Chemistry, 578 S. Shaw Lane, East Lansing, MI 48224-1322. |
| This journal is © The Royal Society of Chemistry 2013 |
