Switchable anionic surfactants for the remediation of oil-contaminated sand by soil washing†
Abstract
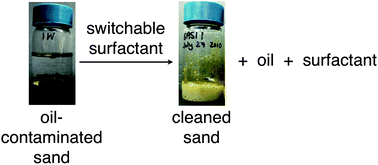
Soil remediation requires technologies to restore contaminated soil to a state that is environmentally acceptable. In most cases, while the soil can be remediated the contaminant itself cannot be reclaimed. However, ex situ soil washing with an aqueous solution of a surfactant would allow easy recovery of the contaminant if the resulting emulsion could be easily and reliably broken. CO2-responsive switchable surfactants that can be switched on and off by CO2 addition and removal would allow facile emulsion breaking, but all reported examples are cationic surfactants. Because soil washing requires anionic surfactants, we have investigated phenolate, benzoate and carboxylate salts as switchable anionic surfactants. These surfactants can indeed be switched on and off with CO2. Three switchable anionic surfactants (sodium 2-nitro-4-((octyloxy)carbonyl)phenolate (1a), sodium 4-(octyloxy) benzoate (3a) and sodium laurate (4a)) and two commercial nonswitchable surfactants (sodium dodecyl sulfate (SDS) and Triton X-100) were evaluated for their ability to wash Ottawa Sand artificially contaminated with North Sea crude oil. At 50 °C, all three switchable surfactants were able to remove North Sea crude oil from sand and have the added feature of facile oil separation and recovery from the wash mixture after CO2 treatment.

 Please wait while we load your content...
Please wait while we load your content...