Abstract
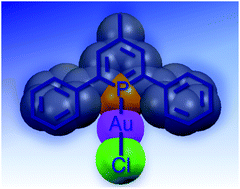
The synthesis of two novel gold(I) complexes with a phosphinine ligand is reported. Reaction of a monodentate phosphinine ligand, 2,6-diphenyl-4-methylphosphorin (Lp; 1), with gold(I) molecular bricks leads to the preparation of two new mononuclear complexes with the general formulae [AuCl(Lp)] (2) and [Au(Lp)2](OTf) (3). The new compounds were spectroscopically characterized by infrared and NMR (1H, 13C and 31P). In addition, the molecular structure of [AuCl(Lp)] (2) was unequivocally ascertained by a single-crystal X-ray diffraction study. Furthermore the UV-Vis absorption and emission properties of these compounds were investigated. These complexes pave the way for the synthesis of a new class of phosphinine gold complexes displaying peculiar properties, which might hold promise as luminescent materials.

 Please wait while we load your content...
Please wait while we load your content...