“On water” highly atom economical and rapid synthesis of a novel class of 3-hydroxy-2-oxindole scaffolds under a catalyst-free and column chromatography-free protocol at room temperature†
Pramod B. Thakur and
Harshadas M. Meshram*
Medicinal Chemistry and Pharmacology Division, CSIR-Indian Institute of Chemical Technology, Hyderabad, 500007, India. E-mail: hmmeshram@yahoo.com; Fax: +91-40-27193275; Tel: +91-40-27191640
First published on 18th December 2013
Abstract
An “on water” highly atom economical and rapid protocol has been developed for the synthesis of a novel class of 3-(2-pyrazolin-5-one) substituted, 3-hydroxy-2-oxindole scaffolds under catalyst-free and column chromatography-free conditions at rt. The generality of the method has been demonstrated by screening a series of isatin electrophiles as well as 2-pyrazolin-5-one derivatives. The developed method is a good example of green synthesis in which straightforward synthesis of a medicinally important 3-hydroxy-2-oxindole framework is executed by employing very mild, simple and handy procedures from readily available starting materials.
Introduction
In the current scenario of chemical synthesis around the world, the term “green chemistry” coined by P. T. Anastas is well acknowledged in both academia and the chemical industry.1 The world-wide synthetic community is already aware of an urgency to development of green chemistry processes, where non-toxic substances are used and the generation of waste can be avoided. Green synthesis protocols not only provide essential atom economy, energy savings, waste reduction, easy workup but also avoid hazardous chemicals.2 Recently, the development of green protocols for the synthesis of highly functionalized motifs having medicinal value has emerged as an attractive area of research.3 However a question that exists is how far the green synthesis protocol could be developed to benefit the synthesis of biologically important molecules.To speak of bioactive molecules, 3-substituted 3-hydroxy-2-oxindole displays a diverse array of natural products as well as pharmaceutically important compounds which possesses a wide range of biological activities.4,5 Selective representative examples are depicted in Fig. 1. Due to such distinction, 3-substituted-3-hydroxy-2-oxindole framework remains an intensively investigated synthetic target.6 Likewise, pyrazolone is pharmaceutically important scaffolds and potential chemotherapeutic agents from the medicinal chemistry point of view possessing imperative biochemical properties.7 Representative examples of compound containing pyrazolone framework include remogliflozin etabonate, phenazone, propyphenazone, ampyrone, edaravone and metamizole.7,8
 | ||
| Fig. 1 Selected representative examples of natural products and pharmaceuticals possessing 3-substituted-3-hydroxy-2-oxidole and pyrazolone structural framework. | ||
The detailed structure–activity relationship studies on3-hydroxy-2-oxindole framework has shown that the biological effects of these compounds are known to vary with the substituent pattern at the C3 position of oxindoles.4e In this context, we envision the C3 substituted molecular scaffold which assimilates 3-hydroxy-2-oxindole as well as pyrazolone framework which might integrate properties of both, and the synergism of both the heterocyclic moieties in a single nucleus may result in the formation of some worthwhile molecules from a biological point of view. Despite the prominence of 3-hydroxy-2-oxindole as well as pyrazolone framework in medicinal chemistry, there is no report on the synthesis of molecular scaffold possessing 3-pyrazolone substituted, 3-hydroxy-2-oxindole framework in the literature to date.
Recently pyrazolones have been explored as a nucleophilic donor in the construction of diversely functionalised heterocyclic scaffolds.9 Surprisingly, investigations on pyrazolones as nucleophilic donors with isatin electrophiles to afford 3-pyrazolone substituted 3-hydroxy-2-oxindole framework remained totally unexplored.9p–v In this context and in continuation of our research work10 on synthesis of 3-substituted 3-hydroxy-2-oxindoles framework, we envisioned that the reaction between isatin and pyrazolone might be readily proceed to afford 3-pyrazolone substituted 3-hydroxy-2-oxindole structural framework (Scheme 1) without the use of any catalyst under certain appropriate reaction conditions due to the fact that the highly reactive β-carbonyl group of isatin is much susceptible to nucleophilic attack.11 We herein wish to report “on water” novel, rapid, atom economical, catalyst-free, and column chromatography-free protocol for synthesis of 3-pyrazolone substituted 3-hydroxy-2-oxindole structural motif at room temperature. We believe that the developed method is one of the good example of an green synthesis protocol in which rapid synthesis of a novel class of medicinally important 3-hydroxy-2-oxindole molecular framework is achieved in quantitative yield using water under catalyst-free and column chromatography-free reaction condition.
 | ||
| Scheme 1 Envisioned synthesis of 3-pyrazolone substituted 3-hydroxy-2-oxindole structural framework. | ||
Results and discussion
As we envisioned the reaction under catalyst-free12 condition, we intended to begin our study using polar solvents. In this context, initially we performed the reaction of isatin 1a with 3-methyl-2-pyrazolin-5-one 2a without any added catalyst in slightly basic polar aprotic solvents like DMF. We were delighted to find that the reaction proceeds under this reaction condition gives 82% conversion of the desired product in 1 h (entry 1, Table 1). However cumbersome aqueous work-up, extraction and column chromatographic purification was found mandatory to get pure product due to which there was a substantial decrease in the yield of isolated product. Similar problem was occurred when we employed DMSO as solvent in the above reaction (entry 2, Table 1). Results with other solvents like CH3CN, THF, MeOH and EtOH were also not much impressive (Table 1, entry 3–6 respectively). We observed that the solvent polarity has an important role in this reaction, however to get rid of isolation problem, we were thinking of employing greener solvent which will give good conversion as well as avoid extra purification steps to get the desired product in pure form. When we talk about the green solvent, water stands as first choice not only because of it is cheap, ready availability and non-toxic nature13 but also due to its ability to enhance the rates and affect the selectivity in various organic transformations due to unique polarity and high hydrogen bonding ability.14 Hence we also attempted the model reaction in water without any additional catalyst and the obtained results were very delightful. When we performed the reaction of isatin 1a (1 mmol) with 3-methyl-2-pyrazolin-5-one 2a (1 mmol) in 5 ml tap water, at the beginning of the reaction, we observe the reaction mixture as a reddish color. As reaction proceeded towards the desired product formation, the color of the reaction mixture was changed from reddish to orange to pale yellow. Finally after 10 min, we found the formation of a thick white color precipitate (Fig. 2). The obtained white color precipitate was filtered, dried and subjected to spectroscopic analysis. We were pleased to recognize the white color solid obtained in the reaction performed in water as a desired product with 100% conversion (entry 7, Table 1).| Entry | Solvent | Time | Conversionb | Isolated yield 3a |
|---|---|---|---|---|
| a Reaction conditions: isatin 1a (1 mmol), 3-methyl-2-pyrazolin-5-one 2a (1 mmol) in 5 ml of solvent at rt.b By 1H NMR analysis.c The pure water was obtained by distillation of tap water and further purified by reverse osmosis.d HPLC grade water was obtained from Sigma-Aldrich.e In 3 ml water.f In 2 ml water.g Under solvent-free reaction condition. | ||||
| 1 | DMF | 1 h | 82 | 71 |
| 2 | DMSO | 1 h | 86 | 73 |
| 3 | CH3CN | 1 h | 51 | 44 |
| 4 | THF | 1 h | 31 | 27 |
| 5 | MeOH | 1 h | 54 | 43 |
| 6 | EtOH | 1 h | 50 | 42 |
| 7 | Tap H2O | 10 min | 100 | 99 |
| 8 | Distilled H2Oc | 10 min | 100 | 99 |
| 9 | HPLC H2Od | 10 min | 100 | 98 |
| 10 | Tap H2Oe | 10 min | 100 | 98 |
| 11 | Tap H2Of | 10 min | 100 | 98 |
| 12 | —g | 24 h | — | — |
| 13 | [bmim]BF4 | 24 h | — | — |
The problem of product isolation associated with solvents like DMSO, DMF was rectified when we employed water in the reaction. It is interesting to note that the progress of the reaction can be monitored just by visualization of the change of color of reaction mixture from red (at the beginning of the reaction) to white (at the end of the reaction). Furthermore the desired product was obtained in highly pure form just by filtration and does not require any column chromatographic purification. As a part of the study we have also performed the reaction in distilled as well as HPLC grade water and similar results were obtained as obtained with tap water (entry 8, 9 Table 1).
Reaction can even perform in less quantity of water to get the desired product however the dilution of reaction mixture requires for the filtration process as it forms a thick precipitate (entry 10, 11). With these observations, we have selected the stirring of isatin 1a (1 mmol) with 3-methyl-2-pyrazolin-5-one 2a (1 mmol) in 5 ml tap water for 10 min at rt as optimized reaction condition to afford quantitative yield of desired product 3a under catalyst-free and column chromatography-free reaction condition (entry 7, Table 1).
With this established optimum condition, we were intended to explore the scope and limitations of the developed protocol with several structurally varied isatins as well as 2-pyrazolin-5-one derivatives and results are incorporated in Table 2. We were delighted to find that, array of isatin derivatives with diverse functional groups were well-tolerated and afforded desired products in quantitative yield with very good purity under optimized condition (Table 2). To be specific, a 3-methyl-2-pyrazolin-5-one 2a reacted smoothly with halogenated isatins like 5-fluoro isatin, 5-chloro isatin, 5-iodo isatin to give the desired products in very good isolated yields (Table 2, entries 2–4).
| a Reaction conditions: isatin 1(a–l) (1 mmol), 2-pyrazolin-5-one derivative 2(a–c) (1 mmol) in 5 ml of tap water at rt and yields are given as an isolated yields. All products 3(a–p) were characterized by NMR, Mass and IR spectroscopic techniques (see ESI file for characterization data and copies of 1H and 13C NMR spectrum). |
|---|
 |
Similarly the reaction of 3-methyl-2-pyrazolin-5-one 2a with other isatin electrophiles like 5-nitro isatin, 5-methoxy isatin and 5-(trifluoromethoxy) isatin afforded desired products 3e, 3f, 3g within very short period of time (Table 2, entry 5, 6, 7 respectively). The reaction was not only successful with isatins having electron withdrawing substituent but also with isatin bearing electron donating group like 5-methyl isatin (Table 2, entry 8). As like mono-substituted isatins, di-substituted isatin like 4,7-dichloro isatin and 5,7-dimethyl isatin reacted in the same way and yielded the desired products under standard reaction condition (Table 2, entry 9 and 16 respectively). The scope of this catalyst-free and column chromatography-free protocol was further extended to N-alkylated isatins like N-methy isatin and N-phenyl isatin which also afforded their respective products 3j, 3k in high yield and purity (Table 2, entry 10, 11). For the general validity of the developed method, we were keen to explore the reactivity of other 2-pyrazolin-5-one nucleophiles with isatin electrophiles. In this context we screened 3-(trifluoromethyl)-2-pyrazolin-5-one 2b and 3-propyl-2-pyrazolin-5-one 2c with different isatins under optimized reaction condition. We observed that the reaction worked well with these substrates also and afforded respective products in very good yield (Table 2, entries 12–16).
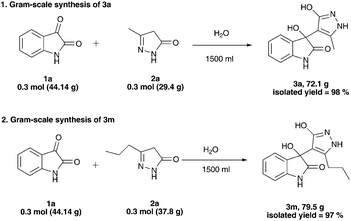
One of the most unique features of this protocol is that, it works just “on water” under catalyst-free or additive-free reaction conditions. Moreover, this transformation is clean and easy to work up which affords desired products in very high yield and purity. Further, in order to demonstrate the scale-up potential of this efficient protocol, we conducted a gram-scale synthesis of 3a and 3m (Scheme 2). We performed the reaction of isatin 1a (0.3 mol, 44.14 g) with 3-methyl-2-pyrazolin-5-one 2a (0.3 mol, 35.14 g) in 1500 ml tap water at rt (entry1, Scheme 2). Similarly we performed the reaction of isatin 1a (0.3 mol, 44.14 g) with 3-propyl-2-pyrazolin-5-one 2b (0.3 mol, 39.94 g) in 1500 ml tap water at rt (entry 2, Scheme 2). The desired products 3a and 3m were obtained in pure form with very good isolated yield (98%, 97% respectively) under this catalyst-free and column chromatography-free protocol.
As a part of the study, to know the effect of substituent's size and position present in 2-pyrazolin-5-one derivative on the success of the reaction, further study was planned. In this regards, we performed the reactions of 3-tert-butyl-1-methyl-2-pyrazolin-5-one 2d with different isatins under optimized reaction condition (Scheme 3). It was observed that the reaction of 3-tert-butyl-1-methyl-2-pyrazolin-5-one 2d with isatins does not proceed towards product formation even after stretching the reaction time up to 24 h. This observation indicates the adverse effect of bulkiness of substituent present in 3-tert-butyl-1-methyl-2-pyrazolin-5-one on the success of reaction. Similarly when we screened 3,4-dimethyl-2-pyrazolin-5-one 2e with different isatins, we does not observed any product formation up to longer reaction time. Furthermore we have also performed the reaction of 2d and 2e with isatin under reflux (100 °C, 24 h) as well as microwave heating condition (101 °C, 140 W, 15 min) but the formation of new product was not observed. Although these observations were surprising, these led us to establish the proper mechanistic sequence of the reaction.
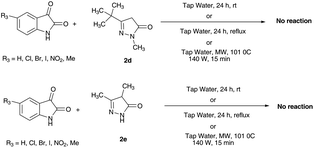 | ||
| Scheme 3 Effect of substituent's size and position present in 2-pyrazolin-5-one derivative on the success of reaction. | ||
The formation of the desired product can be visualized by two possible pathways (Scheme 4). As depicted in path 1, reaction can proceeds by, formation of enolate of 3-methyl-2-pyrazolin-5-one, aldol reaction type addition of enolate of 3-methyl-2-pyrazolin-5-one on isatin 1a to give aldol addition product B, followed by tautomerization of aldol addition product B to give desired product 3a. Likewise, as depicted in path 2, reaction can proceeds by, formation of tautomer of 3-methyl-2-pyrazolin-5-one, nucleophilic addition type reaction of tautomer of 3-methyl-2-pyrazolin-5-one on isatin 1a to form product C, followed by aromatization of product C to give desired product 3a. We believe that the reaction does not proceeds via enolate formation, because we did not observe the formation of aldol addition product A when we treated 3,4-dimethyl-2-pyrazolin-5-one 2e with isatin 1a under optimized reaction condition. With this observation, we proposed the formation of desired products 3a via a mechanistic sequence shown in path 2 in Scheme 4.
 | ||
| Scheme 4 Reasoned mechanistic sequences for the formation of the desired product 3a in this reaction. | ||
Conclusions
In summary, we have developed an “on water” efficient protocol for the synthesis of a novel class of functionalized 3-(2-pyrazolin-5-one derivatives) substituted, 3-hydroxy-2-oxindoles frameworks under catalyst-free and column chromatography-free conditions at rt. The development of this method led to a rapid and general route for the straightforward preparation of medicinally important 3-hydroxy-2-oxindole structural scaffolds in quantitative yields under very mild reaction condition from readily available starting materials. In addition to simplicity, this method has one salient feature in its ability to tolerate a variety of functionalized isatins as well as 2-pyrazolin-5-one derivatives. Such a 3-hydroxy-2-oxindole framework with diverse functionality provides an additional functional handle for further transformations which can be effectively utilized in preparation of a library of pharmaceutically important compounds.Experimental
General procedure for the rapid and atom economical synthesis of 3-(2-pyrazolin-5-one derivatives) substituted, 3-hydroxy-2-oxindole frameworks under catalyst-free and column chromatography free condition using tap water
Mixture of isatin 1(a–l) (1.0 mmol) and 2-pyrazolin-5-one derivatives 2(a–c) (1 mmol) was stirred in 5 ml tap water at room temperature for stipulated time (10 min). The progress of the reaction was monitored by visualisation of the change of color of reaction mixture from red (at the start of the reaction) to white (at the end of the reaction). The progress of the reaction was also confirmed by TLC. The obtained thick white precipitate was filtered and dried to afford the desired product 3(a–p) in very good yield and purity. All products 3(a–p) were characterized by NMR, Mass and IR spectroscopic techniques (see ESI† file for characterization data and copies of 1H and 13C NMR spectrum). (Note that the signal for –OH and –NH proton of pyrazol ring of the products remains undetectable in 1H NMR spectrum which might be due to the rapid exchange ability of such protons.)Spectral data for all the synthesized compounds 3(a–p)
Acknowledgements
PBT thanks CSIR New Delhi for the award of senior research fellowship and Dr Ahmed Kamal (Outstanding Scientist), Head, Medicinal Chemistry and Pharmacology Division, CSIR-Indian Institute of Chemical Technology, Hyderabad for his support and encouragement.Notes and references
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (b) A. S. Matlack, Introduction to Green Chemistry, ed. D. Marcel, New York, 2001 Search PubMed; (c) M. Lancaster, Green Chemistry: An Introductory Text, RSC Editions, London, 2002 Search PubMed.
- (a) A. Kumar and S. Sharma, Green Chem., 2011, 13, 2017–2020 RSC; (b) A. Kumar, G. Gupta and S. Srivastava, Green Chem., 2011, 13, 2459–2463 RSC; (c) A. Kumar, M. K. Gupta and M. Kumar, Green Chem., 2012, 14, 290–295 RSC; (d) K. Tanaka, T. Sugino and F. Toda, Green Chem., 2000, 2, 303–304 RSC; (e) C. L. Raston and J. L. Scott, Green Chem., 2000, 2, 49–52 RSC; (f) P. T. Anastas and T. Williamson, Green Chemistry: Frontiers in Benign Chemical Synthesis and Process, Oxford University Press, Oxford, UK, 1998 Search PubMed; (g) J. McNulty and P. Das, Eur. J. Org. Chem., 2009, 24, 4031–4035 CrossRef; (h) J. McNulty and P. Das, Tetrahedron Lett., 2009, 50, 5737–5740 CrossRef CAS PubMed; (i) J. McNulty, P. Das and D. McLeod, Chem.–Eur. J., 2010, 16, 6756–6760 CrossRef CAS PubMed; (j) P. Das and J. McNulty, Tetrahedron Lett., 2010, 51, 3197–3199 CrossRef CAS PubMed; (k) P. Das, D. McLeod and J. McNulty, Tetrahedron Lett., 2011, 52, 199–201 CrossRef CAS PubMed.
- (a) A. Domling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (b) Multi-component Reaction, ed. J. Zhu and H. Bienayme, Wiley-VCH, Weinheim, 2005 Search PubMed; (c) D. J. Raman and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602–1634 CrossRef PubMed.
- For recent reviews, see: (a) T. Tokunaga, W. E. Hume, T. Umezome, K. Okazaki, Y. Ueki, K. Kumagai, S. Hourai, J. Nagamine, H. Seki, M. Noguchi and R. Nagata, J. Med. Chem., 2001, 44, 4641–4649 CrossRef CAS PubMed; (b) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2964 CrossRef CAS PubMed; (c) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627–646 RSC; (d) T. Kagata, S. Saito, H. Shigemori, A. Ohsaki, H. Kubota and J. Kobayashi, J. Nat. Prod., 2006, 69, 1517–1521 CrossRef CAS PubMed; (e) S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20–38 CrossRef CAS; (f) J. J. Badillo, N. V. Hanhan and A. K. Franz, Curr. Opin. Drug Discovery Dev., 2010, 13, 758–776 CAS; (g) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209–2219 CrossRef CAS; (h) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (i) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36–51 CrossRef CAS; (j) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS.
- (a) P. Hewawasam, N. A. Meanwell, V. K. Gribkoff, S. I. Dworetzky and C. G. Boissard, Bioorg. Med. Chem. Lett., 1997, 7, 1255–1260 CrossRef CAS; (b) N. Boechat, W. B. Kover, V. Bongertz, M. M. Bastos, N. C. Romeiro, M. L. G. Azavedo and W. Wollinger, Med. Chem., 2007, 3, 533–542 CrossRef CAS; (c) P. Hewawasam, M. Erway, S. L. Moon, J. Knipe, H. Weiner, C. G. Boissard, D. J. Post-Munson, Q. Gao, S. Huang, V. K. Gribkoff and N. A. Meanwell, J. Med. Chem., 2002, 45, 1487–1499 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Lee and J. F. Hartwig, J. Org. Chem., 2001, 66, 3402–3415 CrossRef CAS PubMed; (b) A. B. Dounay, K. Hatanaka, J. J. Kodanko, M. Oestreich, L. E. Overman, L. A. Pfeifer and M. M. Weiss, J. Am. Chem. Soc., 2003, 125, 6261–6271 CrossRef CAS PubMed; (c) I. D. Hills and G. C. Fu, Angew. Chem., Int. Ed., 2003, 42, 3921–3924 CrossRef CAS PubMed; (d) B. M. Trost and M. U. Frederiksen, Angew. Chem., Int. Ed., 2005, 44, 308–310 CrossRef CAS PubMed; (e) Y. X. Jia, J. M. Hillgren, E. L. Watson, S. P. Marsden and E. P. Kündig, Chem. Commun., 2008, 4040–4042 RSC; (f) Y. X. Jia and E. P. Kündig, Angew. Chem., Int. Ed., 2009, 48, 1636–1639 CrossRef CAS PubMed.
- (a) M. Himly, B. Jahn-Schmid, K. Pittertschatscher, B. Bohle, K. Grubmayr, F. Ferreira, H. Ebner and C. Ebner, J. Allergy Clin. Immunol., 2003, 111, 882–888 CrossRef CAS; (b) T. Watanabe, S. Yuki, M. Egawa and H. Nishi, J. Pharmacol. Exp. Ther., 1994, 268, 1597–1604 CAS; (c) H. Kawai, H. Nakai, M. Suga, S. Yuki, T. Watanabe and K. I. Saito, J. Pharmacol. Exp. Ther., 1997, 281, 921–927 CAS; (d) T. W. Wu, L. H. Zeng, J. Wu and K. P. Fung, Life Sci., 2002, 71, 2249–2255 CrossRef CAS; (e) M. A. Al-Haiza, S. A. El-Assiery and G. H. Sayed, Acta Pharm. (Zagreb, Croatia), 2001, 51, 251–261 CAS; (f) D. Castagnolo, F. Manetti, M. Radi, B. Bechi, M. Pagano, A. De Logu, R. Meleddu, M. Saddi and M. Botta, Bioorg. Med. Chem., 2009, 17, 5716–5721 CrossRef CAS PubMed; (g) F. Moreau, N. Desroy, J. M. Genevard, V. Vongsouthi, V. Gerusz, G. Le Fralliec, C. Oliveira, S. Floquet, A. Denis, S. Escaich, K. Wolf, M. Busemann and A. Aschenbsenner, Bioorg. Med. Chem. Lett., 2008, 18, 4022–4026 CrossRef CAS PubMed; (h) E. A. M. Badawey and I. M. El-Ashmawey, Eur. J. Med. Chem., 1998, 33, 349–361 CrossRef CAS; (i) F. A. Pasha, M. Muddassar, M. M. Neaz and S. J. Cho, J. Mol. Graphics Modell., 2009, 28, 54–61 CrossRef CAS PubMed; (j) C. E. Rosiere and M. I. Grossman, Science, 1951, 113, 651–653 CAS; (k) D. M. Bailey, P. E. Hansen, A. G. Hlavac, E. R. Baizman, J. Pearl, A. F. Defelice and M. E. Feigenson, J. Med. Chem., 1985, 28, 256–260 CrossRef CAS; (l) P. M. S. Chauhan, S. Singh and R. K. Chatterjee, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1993, 32, 858–861 Search PubMed; (m) P. Gunasekaran, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2011, 46, 4530–4536 CrossRef CAS PubMed; (n) H. Shiohara, H. Fujikura, N. Fushimi, F. Ito and M. Isaji, PCT Int. Appl. WO 2002098893, 2002; Chem. Abstr., 2003, 138, 2491724925; (o) N. Foloppe, L. M. Fisher, R. Howes, A. Potter, A. G. S. Robertson and A. E. Surgenor, Bioorg. Med. Chem., 2006, 14, 4792–4802 CrossRef CAS PubMed; (p) D. A. Barawkar, A. Meru, A. Bandyopadhyay, A. Banerjee, A. M. Deshpande, C. Athare, C. Kodurum, G. Khose, J. Gundu, K. Mahajan, P. Patil, S. R. Kandalkar, S. Niranjan, S. Bhosale, S. De, S. Mukhopadhyay, S. Chaudhary, S. Koul, U. Singh, A. Chugh, V. P. Palle, K. A. Mookhtiar, J. Vacca, P. K. Chakravarty, R. P. Nargund, S. D. Wright, S. Roy, M. P. Graziano, S. B. Singh, D. Cully and T. Q. Cai, ACS Med. Chem. Lett., 2011, 2, 919–923 CrossRef CAS; (q) D. A. Barawkar, A. Bandyopadhyay, A. Deshpande, S. Koul, S. Kandalkar, P. Patil, G. Khose, S. Vyas, M. Mone, S. Bhosale, U. Singh, S. De, A. Meru, J. Gundu, A. Chugh, V. P. Palle, K. A. Mookhtiar, J. P. Vacca, P. K. Chakravarty, R. P. Nargund, S. D. Wright, S. Roy, M. P. Graziano, D. Cully, T. Q. Cai and S. B. Singh, Bioorg. Med. Chem. Lett., 2012, 22, 4341–4347 CrossRef CAS PubMed.
- For reviews on pyrazolines, see: (a) A. Levai, J. Heterocycl. Chem., 2002, 39, 1–13 CrossRef CAS; (b) M. Kissane and A. R. Maguire, Chem. Soc. Rev., 2010, 39, 845–883 RSC; (c) C. H. Kuchenthal and W. Maison, Synthesis, 2010, 5, 719–740 Search PubMed.
- For selected examples, see: (a) R. K. Boeckman Jr, J. E. Reed and P. Ge, Org. Lett., 2001, 3, 3651–3653 CrossRef CAS PubMed; (b) M. Abass and B. B. Mostafa, Bioorg. Med. Chem., 2005, 13, 6133–6144 CrossRef CAS PubMed; (c) M. S. Chande, P. A. Barve and V. Suryanarayan, J. Heterocycl. Chem., 2007, 44, 49–53 CrossRef CAS; (d) G. Mariappan, B. P. Saha, N. R. Bhuyan, P. R. Bharti and D. Kumar, J. Adv. Pharm. Technol. Res., 2010, 1, 260–267 CrossRef CAS PubMed; (e) R. Ma, J. Zhu, J. Liu, L. Chen, X. Shen, H. Jiang and J. Li, Molecules, 2010, 15, 3593–3601 CrossRef CAS PubMed; (f) Y. H. Liao, W. B. Chen, Z. J. Wu, X. L. Du, L. F. Cun, X. M. Zhang and W. C. Yuan, Adv. Synth. Catal., 2010, 352, 827–832 CrossRef CAS; (g) S. Gogoi and C. G. Zhao, Tetrahedron Lett., 2009, 50, 2252–2255 CrossRef CAS PubMed; (h) M. Radi, V. Bernardo, B. Bechi, D. Castagnolo, M. Pagano and M. Botta, Tetrahedron Lett., 2009, 50, 6572–6575 CrossRef CAS PubMed; (i) S. Gogoi, C. G. Zhao and D. Ding, Org. Lett., 2009, 11, 2249–2252 CrossRef CAS PubMed; (j) Z. G. Yang, Z. Wang, S. Bai, X. Liu, L. Lin and X. Feng, Org. Lett., 2011, 13, 596–599 CrossRef CAS PubMed; (k) A. N. Alba, A. Zea, G. Valero, T. Calbet, M. Font-Bardia, A. Mazzanti, A. Moyano and R. Rios, Eur. J. Org. Chem., 2011, 1318–1325 CrossRef CAS; (l) Z. Wang, Z. Yang, D. Chen, X. Liu, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2011, 50, 4928–4932 CrossRef CAS PubMed; (m) M. R. Rohman, H. Mecadon, A. T. Khan and B. Myrboh, Tetrahedron Lett., 2012, 53, 5261–5264 CrossRef PubMed; (n) J. W. Wu, F. Li, Y. Zheng and J. Nie, Tetrahedron Lett., 2012, 53, 4828–4831 CrossRef CAS PubMed; (o) P. P. Ghosh, G. Pal, S. Paul and A. R. Das, Green Chem., 2012, 14, 2691–2698 RSC; (p) Y. Liu, Z. Ren, W. Cao, J. Chen, H. Deng and M. Shao, Synth. Commun., 2011, 41, 3620–3626 CrossRef CAS; (q) M. N. Elinson, A. I. Ilovaisky, V. M. Merkulova, G. I. Nikishin and T. A. Zaimovskaya, Mendeleev Commun., 2012, 22, 143–144 CrossRef CAS PubMed; (r) R. G. Redkin, L. A. Shemchuk, V. P. Chernykh, O. V. Shishkin and S. V. Shishkina, Tetrahedron, 2007, 63, 11444–11450 CrossRef CAS PubMed; (s) F. F. Abdel, R. A. Mekheimer, M. M. Mashaly and E. K. Ahmed, Collect. Czech. Chem. Commun., 1994, 59, 1235–1240 CrossRef; (t) K. C. Joshi, R. T. Pardasani, A. Dandia and S. Bhagat, Heterocycles, 1991, 32, 1491–1498 CrossRef CAS PubMed; (u) N. Hussain, R. Dangi, C. K. Sharma and G. L. Talesara, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2011, 50, 885–889 Search PubMed; (v) S. A. Metwally, M. I. Younes and H. H. Abbas, Acta Chim. Hung., 1989, 126, 591–597 CAS.
- (a) H. M. Meshram, P. Ramesh, B. C. Reddy, B. Shreedhar and J. S. Yadav, Tetrahedron, 2011, 67, 3150–3155 CrossRef CAS PubMed; (b) H. M. Meshram, D. A. Kumar, B. C. Reddy and P. Ramesh, Synth. Commun., 2010, 40, 39–45 CrossRef CAS; (c) H. M. Meshram, P. Ramesh, A. S. Kumar and A. Swetha, Tetrahedron Lett., 2011, 52, 5862–5864 CrossRef CAS PubMed; (d) H. M. Meshram, R. N. Nageswara, R. L. Chandrasekhara and S. N. Kumar, Tetrahedron Lett., 2012, 53, 3963–3966 CrossRef CAS PubMed; (e) P. B. Thakur, K. Sirisha, A. V. S. Sarma, J. B. Nanubolu and H. M. Meshram, Tetrahedron, 2013, 69, 6415–6423 CrossRef CAS PubMed.
- (a) F. D. Popp, Adv. Heterocycl. Chem., 1975, 18, 1–58 CrossRef CAS; (b) J. F. M. da Silva, S. J. Garden and A. C. Pinto, J. Braz. Chem. Soc., 2001, 12, 273–324 CrossRef CAS PubMed.
- (a) G. Choudhary and R. K. Peddinti, Green Chem., 2011, 13, 276–282 RSC; (b) S. Kumar, P. Sharma, K. K. Kapoor and M. S. Hundal, Tetrahedron, 2008, 64, 536–542 CrossRef CAS PubMed; (c) J. Zhang, Z. Cui, F. Wang, Y. Wang, Z. Miao and R. Chen, Green Chem., 2007, 9, 1341–1345 RSC; (d) A. Kumar, M. Kumar and M. K. Gupta, Green Chem., 2012, 14, 2677–2681 RSC; (e) G. R. Qu, H. L. Zhang, H. Y. Niu, Z. K. Xue, X. X. Lva and H. M. Guo, Green Chem., 2012, 14, 1877–1879 RSC; (f) F. E. A. Van Waes, J. Drabowicz, A. Cukalovica and C. V. Stevens, Green Chem., 2012, 14, 2776–2779 RSC; (g) F. Alonso, Y. Moglie, G. Radivoy and M. Yusa, Green Chem., 2012, 14, 2699–2702 RSC; (h) M. Jereb, Green Chem., 2012, 14, 3047–3052 RSC; (i) J. Liu, M. Lei and L. Hu, Green Chem., 2012, 14, 2534–2539 RSC; (j) B. D. Bala, S. M. Rajesh and S. Perumal, Green Chem., 2012, 14, 2484–2490 RSC; (k) R. Pelagalli, I. Chiarotto, M. Feroci and S. Vecchio, Green Chem., 2012, 14, 2251–2255 RSC; (l) C. Zhang, L. Zhanga and N. Jiao, Green Chem., 2012, 14, 3273–3276 RSC; (m) Z. N. Tisseh, M. Dabiri, M. Nobahar, H. R. Khavasi and A. Bazgir, Tetrahedron, 2012, 68, 1769–1773 CrossRef CAS PubMed.
- (a) H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114–120 CrossRef CAS; (b) C. J. Li and C. Liang, Chem. Soc. Rev., 2006, 35, 68–82 RSC.
- (a) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed; (b) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed; (c) V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743–754 RSC; (d) G. L. Khatik, R. Kumar and A. K. Chakraborti, Org. Lett., 2006, 8, 2433–2436 CrossRef CAS PubMed; (e) N. Azizi and M. R. Saidi, Org. Lett., 2005, 7, 3649–3651 CrossRef CAS PubMed; (f) N. Azizi, F. Aryanasab, L. Torkiyan, A. Ziyaei and M. R. J. Saidi, J. Org. Chem., 2006, 71, 3634–3635 CrossRef CAS PubMed; (g) B. C. Ranu and S. Banerjee, Tetrahedron Lett., 2007, 48, 141–143 CrossRef CAS PubMed; (h) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data as well as copies of 1H and 13C NMR spectrum. See DOI: 10.1039/c3ra46613b |
| This journal is © The Royal Society of Chemistry 2014 |