Facile synthesis of ZnWO4 nanowall arrays on Ni foam for high performance supercapacitors
Bingkun Guan,
Lingling Hu,
Guanhua Zhang,
Di Guo,
Tao Fu,
Jidong Li,
Huigao Duan,
Chengchao Li and
Qiuhong Li*
Key Laboratory for Micro-Nano Optoelectronic Devices of Ministry of Education, State Key Laboratory for Chemo/Biosensing and Chemometrics, Hunan University, Changsha, 410082, P. R. China. E-mail: liqiuhong2004@hotmail.com; Fax: +86 07 31 88822137; Tel: +86 0731 88664019
First published on 7th November 2013
Abstract
In this report, ZnWO4 nanowall arrays (NWAs) were grown on Ni foam by a hydrothermal route and investigated for application in supercapacitors for the first time. The resulting NWAs were analyzed by using X-ray diffraction spectroscopy, scanning electron microscopy, and transmission electron microscopy. ZnWO4 NWAs exhibited high electrochemical property with a specific capacitance of 2.5 F cm−2 at a charge and discharge current density of 20 mA cm−2 (1250 F g−1 at current density of 10 A g−1). Furthermore, they showed an excellent cycling ability at different current densities up to 100 mA cm−2, and 92% (2.3 F cm−2) of the initial capacitance remained after 4000 cycles. The open network structure consisting of interconnected ZnWO4 NWAs directly grown on current collectors is advantageous for electron transport and electrolyte diffusion which can facilitate the electrochemical reaction. Our work not only demonstrated a facile hydrothermal method for ZnWO4 NWAs directly grown on Ni foam, but also revealed ZnWO4 to be a promising electrode for high-property supercapacitors.
Introduction
In recent years, one of the great challenges has been to provide low-cost and environmentally friendly high-power energy resources,1,2 owing to increasing concern about environmental issues and the depletion of fossil fuels. Supercapacitors, a promising energy storage device for electrical energy,3 offer ideally high power density, fast recharge ability and long cycle life for time-dependent power demands for modern electronics and power systems.3–6 The charge and discharge processes of supercapacitors are much faster than lithium-ion batteries (LIBs), and their energy density is higher than conventional dielectric capacitors.2,7,8 The performance of supercapacitors is greatly determined by the properties of electrode materials.9 As electrode materials for supercapacitors, carbon based materials and many transition metal oxides have been widely studied. However, due to low specific capacity of carbon based materials, they can hardly meet the growing requirements for electric vehicles and renewable sources of energy,5,6 and high cost of some transition metal oxides also limited their practical use in supercapacitors. Therefore, a major challenge for supercapacitors is to develop new capacitive materials with not only high energy density but also long cycle life for promising practical applications in high-power electric vehicles and portable electronic devices.5,6,10Nowadays, nanostructured materials have been intensively studied in many fields such as LIBs, capacitors, catalysis and bionanotechnology etc.11–14 Many kinds of promising electrode materials have been investigated for supercapacitors, including some transition metal oxides (MnO2,15 Co3O4,16,17 NiO,18,19 etc.). Recently, three-dimensional (3D) nanostructures grown directly on current collectors are attractive for a charge carrier transport and stress relaxation. For example, ternary NiCo2O4,20,21 some 3D hybrid nanostructures including ZnO@NiHN,22 MnO2@NiO,23 CoO@NiHON,24 Co3O4@MnO2,25 Co3O4@NiCo2O4,26 and some metal oxides grown on three dimensional graphene foam such as, graphene/ZnO,27 graphene–MnO228 and graphene/Co3O429 had significantly enhanced both the capacitance and durability of the electrodes. The structure can shorten the path length of ion diffusion and promote the contribution of the electro-active materials to capacitance efficiently. Now, construction of nanowall arrays (NWAs) directly grown on a current collecting substrate (such as Ni2S3,30 CoO,31 NiO32,33 and ZnO34 NWAs), was found to be an effective route to further improve the electrochemical performance because of the following advantages. Firstly, the open space between NWAs allows electrolyte easily getting into the inner region of the electrode, reducing the electrolyte's resistance. Secondly, the NWAs on substrate with a robust adhesion provide numerous fast electron-transport accesses to the current collector, not only avoiding using the polymer binder, but also decreasing polarization and improving the rate capability of electrode materials. And finally, there is no need to incorporate binders and carbon black for the electrode to overcome additional undesirable interfaces and defects, resulting in low electrolyte diffusion efficiency.35 In this work, ZnWO4 was also nanowall arrays structure. In addition, the metal tungstate is a good group of materials because of their similarity to ternary oxides and the high oxidation state observed in the tungsten atom. The tungstate family is an important group of inorganic materials with applications in many fields. This family of compounds can crystallize in either the scheelite structure or the wolframite structure.36 As a member of the metal tungstate family, ZnWO4 has significantly enhanced the performance of Li-ion batteries, because both Zn and W are electrochemically active metals with respect to Li metal. And tungstate oxide is an excellent pseudocapacitive material because of its high-specific-area and high-conductivity. For example, Yoon et al. obtained mesoporous WO3−x and investigated its applications in SCs and LIBs.37,38 So, the ZnWO4 NWAs can be a promising electrode material for the development of high-property supercapacitors. However, to the best of our knowledge, there are no reports on the fabrication of ZnWO4 NWAs on substrates that can be promising supercapacitors.
In this report, a new ZnWO4 NWAs structure on Ni foam was developed by a simple hydrothermal route, and its application in supercapacitors was systematically studied. The NWAs electrodes exhibited good cycling stability and high rate capability during long term cycling at different current densities. It showed a high area specific capacitance (ASC) of 2.5 F cm−2 at 20 mA cm−2, long cycle life up to 4000 cycles in a 3 M KOH aqueous electrolyte. Importantly, ZnWO4 is environmentally friendly low-cost transition metal oxide, with both its constituent elements being relatively earth-abundant. Thus, ZnWO4 is a promising electrode material for the development of high-property supercapacitors.
Experimental section
Fabrication of ZnWO4 nanowalls on Ni foam
In the experiment, all the analytical-grade chemicals were used without any purification process. The deionized water was Millipore Milli-Q grade with a resistivity larger than 18 MΩ cm−1. Ni foam supported-ZnWO4 defoliation-like nanowalls were prepared via a template-free growth method. After degreased with acetone, the Ni foam substrate (specific surface area, 0.00286 m2 g−1; porosity ≥95%) was etched with 6 M HCl in an ultrasound for 15 min, and then washed with deionized water and ethanol for 20 min, respectively. After dried, the Ni foam was placed standing against the wall of Teflon-lined autoclave (50 mL). For the hydrothermal (HT) method, zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 2 mmol, Tianjin Chemicals, 99.5%) and an equal amount of sodium tungstate dehydrate (Na2WO4·2H2O, 2 mmol, Tianjin Chemicals, 99.0%) were dissolved separately in 20 mL of distilled water with constant magnetic stirring. After stirring for 30 min, the zinc nitrate solution was added slowly to the sodium tungstate solution. Then, ammonium fluoride (NH4F, 4 mmol, Tianjin Chemicals, 96.0%) was added to the mixture, again with constant magnetic stirring for 30 min. The mixture was then transferred into the above Teflon-lined stainless steel autoclave, which was sealed and maintained at 120 °C for 12 h in an electric oven and then cooled to ambient temperature without any additional cooling system. The final product supported on the Ni foam was washed with distilled water and absolute alcohol several times with the assistance of ultrasonication and dried at 60 °C for 6 h.The product supported on the Ni foam was put into a quartz tube in Ar gas and annealed at 350 °C for 2 h with a heating rate of 1.46 °C min−1. Then the sample was cooled down to room temperature naturally to obtain ZnWO4 NWAs.
Characterization
The crystal structure of the samples was determined by X-ray diffraction (XRD, Cu Kα irradiation; λ = 1.5418 Å) with a SIEMENS D5000 X-ray diffractometer at a scan rate of 8° min−1 in the 2θ range from 10° to 80°. The morphology and microstructure of the synthesized sample were characterized by a scanning electron microscopy (SEM, Hitachi S4800) and a transmission electron microscope (TEM; JEOL-2010 with an accelerating voltage of 200 kV).Electrochemical measurements
All Electrochemical measurements were made in a conventional three-electrode cell containing 3 M KOH aqueous solution as the electrolyte. The ZnWO4 NWAs on Ni foam (1 cm × 1 cm) acted as the working electrode, and a platinum plate and a saturated calomel electrode were used as counter and reference electrodes, respectively. The cyclic voltammetry (CV) measurements and galvanostatic charge–discharge tests were performed on a CHI660e electrochemical workstation (Chenhua, Shanghai).Results and discussion
Characterization of the products
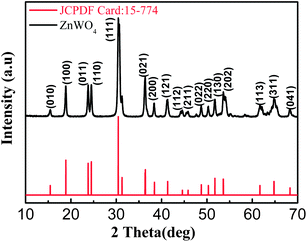
The composition and phase structure of the products were first analyzed by XRD. In order to eliminate the strong impact of Ni foam substrate on the XRD diffraction peaks signals, the product powder was scratched from Ni foam. The corresponding XRD pattern was shown in Fig. 1.From this figure, all the diffraction peaks can be indexed to the ZnWO4 with the standard card (JCPDS card no. 73-0554). No peak was observed from other crystallized phases, which also indicated the formation of pure ZnWO4 product.
The morphologies and microstructures of the ZnWO4 NWAs were examined by SEM, as shown in Fig. 2. From Fig. 2(a), the bare Ni foam (left) was light gray. It turned to dark gray after the hydrothermal process (middle), and then to black (right) after heat treatment at 350 °C for 2 h. In the experiment, Ni foam was chosen as a substrate because of its good electrical conductivity and favorable three-dimensional (3D) hierarchical macroporous structure.39 Fig. 2(b) shows that ZnWO4 NWAs are supported on the net-like 3D porous Ni foam.
 | ||
| Fig. 2 (a) Photographs of Ni foam substrate (left), samples grown on Ni foam before (middle) and after (right) annealing; (b–e) low and (f) high magnification SEM images of ZnWO4 NWAs on the Ni foam. | ||
Fig. 2(c) shows the low-magnification SEM image, which clearly shows that the products with high density are uniformly grown on the surface of the Ni foam. Fig. 2(d) and (e) show top-view SEM images. As observed, the ZnWO4 NWAs vertically stand on the surface of Ni foam, and form an open-up network structure by interconnected nanowalls. Thus, the NWAs can provide an effective channel for the electrolyte when used as electrodes for supercapacitors. From the high magnification SEM image Fig. 2(f), the tablet of a single nanowall is very thin.
Transmission electron microscopy (TEM) analysis was carried out to further investigate the crystal structure and morphologies of the ZnWO4 NWAs. Fig. 3(a) shows the typical TEM image after a strong ultrasonic vibration in ethanol, and the inset in Fig. 3(a) is an individual and complete nanowall. The inset shows hexagon shape of a complete nanowall.
 | ||
| Fig. 3 (a) TEM image of the ZnWO4 nanowalls, the inset is an individual and complete nanowall; (b) the corresponding SAED pattern on an individual nanowall; (c) HRTEM image of the resulting ZnWO4 nanowalls, inset in Fig. 3(c) is TEM image of the ZnWO4 nanowalls; (d) corresponding HRTEM image of the region highlighted by a red rectangle in (c). | ||
From Fig. 3(b), the corresponding selected-area electron diffraction (SAED) pattern is also performed for the area of ZnWO4 nanowall marked by a red oval in Fig. 3(a). The high-resolution TEM (HRTEM) image (Fig. 3(d)) was taken from the parts of the region highlighted by a red rectangle in Fig. 3(c). From this image, the interplanar spacing is measured to be about 0.363 nm, which is in good agreement with the standard d-spacing of the (110) plane of ZnWO4 and the inset in Fig. 3(c) reveals hexagon shape of a nanowall with diameter of about 100 nm.
Most remarkably, mass loading of active material per unit area and a tight binding between active material and substrate are two important factors for supercapacitor.40,41 NH4F plays an essential role in the hydrothermal process for achieving these two goals. Without NH4F, the products can be removed easily by several minutes of ultrasonication. The SEM images were shown in Fig. 4.
As can be seen from Fig. 2, when ammonium fluoride was added to the mixture of zinc nitrate hexahydrate and sodium tungstate dehydrate, the ZnWO4 NWAs had a strong adhesion to the Ni foam substrate and almost could not be dislodged from Ni foam after 60 min of ultrasonication. But without NH4F the products could be removed easily by 30 min of ultrasonication as shown in Fig. 4. This phenomenon evidently revealed the fact that NH4F was largely associated with adhesion between the substrate and the nano-arrays which was in good accordance with previously reported literature.42
Electrochemical characteristics
In order to evaluate the electrochemical performance of ZnWO4 NWAs supported on Ni foam, cyclic voltammetry (CV) and chronopotentiometry (CP) measurements were conducted in a three-electrode system with 3 M KOH aqueous solution as the electrolyte. Fig. 5(a) shows cyclic voltammetry (CV) of self-supported ZnWO4 NWAs on Ni foam within the potential range of 0.05–0.65 V at various scan rates ranged from 5 to 50 mV s−1. The CV curves at different scan rates shows a pair of strong redox peaks, which indicates that the capacitance characteristics are mainly governed by Faradaic redox reactions. At a low scan rate of 5 mV s−1, the anodic peak at 0.48 V is due to the oxidation process, while the cathodic peak at about 0.29 V is related to its reverse process.All curves still exhibit a similar shape with the increase of the scan rates, which reveal the ideal capacitive behaviors of the ZnWO4 NWAs on Ni foam. Obviously, with the increase of the scan rate from 5 to 50 mV s−1 the anodic and the cathodic peaks shift to higher and to lower potentials, respectively. It may be that the charge transfer kinetics is the limiting step of the reaction, which leads to a lower specific capacitance. In order to get more information about the capacitive property of the ZnWO4 NWAs electrodes, the galvanostatic charge–discharge measurements were conducted at various current densities with the voltage range between 0 and 0.6 V. The galvanostatic discharge curves at various current densities (20, 30, 50, and 100 mA cm−2) are shown in Fig. 5(b). The discharge curves in Fig. 5(b) showed a significant deviation from a straight and flat line, indicating that the capacitance mainly comes from the faradic redox reactions, so the existence of plateaus in the discharge curves suggests the typical pseudocapacitive characteristics, which is consistent with the CV curves (Fig. 5(a)). The mass loading of Zn2WO4 NWAs on Ni foam is about 2 mg cm−2. The ASC of ZnWO4 NWAs on Ni foam electrode is calculated from discharge time according to the following equation:
The ASC of the Ni foam-supported ZnWO4 NWAs can be calculated to be 2.5, 2.2, 1.9, and 1.1 F cm−2 at different current densities of 20, 30, 50, and 100 mA cm−2, respectively. The ASC gradually decreases with the increase of discharge current density due to the incremental potential drop and the relatively insufficient active material involved in redox reaction under higher current densities. As can be seen from Fig. 5(c), at a high current density of 100 mA cm−2 (50 A g−1), the Ni foam-supported ZnWO4 NWAs still showed a high capacitance of 1.1 F cm−2 (550 F g−1). Clearly, our ZnWO4 NWAs demonstrate superior high-rate capacitance at high current density, revealing the ZnWO4 NWAs have potential application in high property supercapacitors. Furthermore, the specific capacitance reported here is much higher than those reported WO3 and WO3 composite materials. As can be seen from Table 1, WO3−x @MnO2 NWs grown on carbon cloth (341 F g−1 at a 10 mV s−1 scan rate),43 and the WO3−x@Au@MnO2 NWs grown on carbon cloth (588 F g−1 at a 10 mV s−1 scan rate).43 even higher than those previously reported directly grown pseudocapacitive array nanoarchitectures, such as CoMoO4 nanoplate arrays (1.26 F cm−2 at 4 mA cm−2),35 Co3O4–MnO2 nanowire/nanosheet core–shell arrays (0.56 F cm−2 at 11.25 mA cm−2),25 MnO2–NiO core–shell nanoflakes arrays (0.35 F cm−2 at 9.5 mA cm−2),23 and etc.
Long cycle life is another critical requirement for practical applications of a supercapacitor. The cycle stability of Ni foam supported ZnWO4 NWAs electrodes and bare Ni foam electrodes were tested in the range of 0–0.6 V in 3 M KOH solution by repeated charge–discharge processes. Fig. 5(d) shows the ZnWO4 NWAs electrodes exhibits excellent electrochemical stability and good rate capability. During the first 1500 cycles at 20 mA cm−2, the ASC maintains at 2.5 F cm−2 without noticeable decrease. After continuous cycling at 30, 50, and 100 mA cm−2 for respective 500 times, the current densities turned back to 20 mA cm−2, and 92% (2.3 F cm−2) of the initial capacitance was still remained and maintained for another 1000 cycles without noticeable diminishment. As a substrate, 3D Ni foam enables efficient charge transport and accessible diffusion of electrolyte. The cycle characteristic of the bare Ni foam as electrode under the same test conditions was also presented in Fig. 5(d). As can be seen from the red line, at a current density of 20 mA cm−2, the specific capacitance of Ni foam substrate only contributes 0.25 F cm−2 (7.2 F g−1), which can be regarded that the contribution of Ni foam is negligible.
Above all, such good electrochemical property of ZnWO4 NWAs on Ni foam can be attributed to the unique hierarchical structure. Firstly, the Ni foam substrate with micro holes and zigzag flow channels provides numerous fast electron-transport accesses to the current collector. Secondly, the open network structure constituted of interconnected nanowalls is advantageous for electron transport and electrolyte diffusion which can accelerate the electrochemical reaction. Thirdly, each nanowall is directly grown on Ni foam, so there is no need for the binders or carbon black, which can overcome additional undesirable interfaces and defects, and thus ensure all NWAs to participate in the electrochemical reaction, enhancing the utilization of active materials. As a result, ZnWO4 NWAs can exhibit so good properties.
Conclusions
In summary, a novel ZnWO4 NWAs material on Ni foam was synthesized for application in supercapacitors by a hydrothermal route. It was demonstrated that the ZnWO4 NWAs exhibited good electrochemical properties with a high areal capacitance 2.5 F cm−2 (1250 F g−1) at 20 mA cm−2 (10 A g−1) and 2.2 F cm−2 (1100 F g−1) at 30 mA cm−2 (15 A g−1). The electrode also showed desirable rate property and electrochemical stability up to 100 mA cm−2. Moreover, the electrodes could maintain good property stability over 4000 cycles and no obvious decrease was observed at 20 mA cm−2. Such good electrochemical property could be mainly ascribed to the unique ZnWO4 NWAs structure. The open-up network structure formed by interconnected nanowalls could be a promising candidate for high property supercapacitors. In term of their ease of fabrication, the ZnWO4 NWAs grown on Ni foam will hold promise for application in supercapacitors.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (Grant no. 21003041, 61376073), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20120161110016), the Hunan Provincial Natural Science Foundation of China (Grant no. 11JJ7004), and the Hunan Provincial Major Project of Science and Technology Department (Grant no. 2012TT1004).Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS.
- B. H. Qu, H. X. Li, M. Zhang, L. Mei, L. B. Chen, Y. G. Wang, Q. H. Li and T. H. Wang, Nanoscale, 2011, 3, 4389 RSC.
- J. R. Miller and P. Simon, Science, 2008, 321, 651 CrossRef CAS PubMed.
- T. Stimpfling and F. Leroux, Chem. Mater., 2010, 22, 974 CrossRef CAS.
- X. Zhao, B. M. Sanchez, P. J. Dobson and P. S. Grant, Nanoscale, 2011, 3, 839 RSC.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS PubMed.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- R. Marom, S. F. Amalraj, N. Leifer, D. Jacob and D. Aurbach, J. Mater. Chem., 2011, 21, 9938 RSC.
- E. Kim, D. Son, T. G. Kim, J. Cho, B. Park, K. S. Ryu and S. H. Chang, Angew. Chem., 2004, 116, 6113 CrossRef.
- L. Espinal, S. L. Suib and J. F. Rusling, J. Am. Chem. Soc., 2004, 126, 7676 CrossRef CAS PubMed.
- A. R. Armstrong and P. G. Bruce, Nature, 1996, 381, 499 CrossRef CAS.
- S. Devaraj and N. Munichandraiah, J. Phys. Chem. C, 2008, 112, 4406 CAS.
- H. J. Huang and X. Wang, Nanoscale, 2011, 3, 3185 RSC.
- T. Y. Wei, C. H. Chen, H. C. Chien, S. Y. Lu and C. C. Hu, Adv. Mater., 2010, 22, 347 CrossRef CAS PubMed.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, RSC Adv., 2012, 2, 1835 RSC.
- J. Y. Lee, K. Liang, K. H. An and Y. H. Lee, Synth. Met., 2005, 150, 153 CrossRef CAS PubMed.
- Y. G. Wang and Y. Y. Xia, Electrochim. Acta, 2006, 51, 3223 CrossRef CAS PubMed.
- D. Carriazo, J. Patinño, M. C. Gutiérrez, M. L. Ferrer and F. D. Monte, RSC Adv., 2013, 3, 13690 RSC.
- C. Z. Yuan, J. Y. Li, L. R. Hou, X. G. Zhang, L. F. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS.
- J. P. Liu, C. W. Cheng, W. W. Zhou, H. X. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436 RSC.
- J. P. Liu, J. Jiang, M. Bosmanc and H. J. Fan, J. Mater. Chem., 2012, 22, 2419 RSC.
- C. Guan, J. P. Liu, C. W. Cheng, H. X. Li, X. L. Li, W. W. Zhou, H. Zhang and H. J. Fan, Energy Environ. Sci., 2011, 4, 4496 CAS.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- G. H. Zhang, T. H. Wang, X. Z. Yu, H. N. Zhang, H. G. Duanand and B. A. Lu, Nano Energy, 2013, 2, 586 CrossRef CAS PubMed.
- X. C. Dong, Y. F. Cao, J. Wang, M. B. Chan-Park, L. H. Wang, W. Huang and P. Chen, RSC Adv., 2012, 2, 4364 RSC.
- J. Yan, Z. J. Fan, T. Wei, W. Z. Qian, M. L. Zhang and F. Wei, Carbon, 2010, 48, 3825 CrossRef CAS PubMed.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, W. Huang and P. Chen, ACS Nano, 2010, 6, 3206 CrossRef PubMed.
- C. H. Lai, K. W. Huang, J. H. Cheng, C. Y. Lee, W. F. Lee, C. T. Huang, B. J. Hwang and L. J. Chen, J. Mater. Chem., 2009, 19, 7277 RSC.
- J. Jiang, J. P. Liu, R. M. Ding, X. X. Ji, Y. Y. Hu, X. Li, A. Z. Hu, F. Wu, Z. H. Zhu and X. T. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 99 CAS.
- B. Varghese, M. V. Reddy, Y. W. Zhu, C. S. Lit, T. C. Hoong, G. V. S. Rao, B. V. R. Chowdari, A. T. S. Wee, C. T. Lim and C. H. Sow, Chem. Mater., 2008, 20, 3360 CrossRef CAS.
- X. H. Huang, J. P. Tu, X. H. Xia, X. L. Wang, J. Y. Xiang, L. Zhang and Y. Zhou, J. Power Sources, 2009, 188, 588 CrossRef CAS PubMed.
- M. M. Brewster, M.-Y. Lu, S. K. Lim, M. J. Smith, X. Zhou and S. Gradecak, J. Phys. Chem. Lett., 2011, 2, 1940 CrossRef CAS.
- D. Guo, H. M. Zhang, X. Z. Yu, M. Zhang, P. Zhang, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2013, 1, 7247 CAS.
- R. J. D. Tilley, Int. J. Refract. Met. Hard Mater., 1995, 13, 93 CrossRef CAS.
- S. Yoon, E. Kang, J. K. Kim, C. W. Lee and J. Lee, Chem. Commun., 2011, 47, 1021 RSC.
- S. Yoon, C. Jo, S. Y. Noh, C. W. Lee, J. H. Song and J. Lee, Phys. Chem. Chem. Phys., 2011, 13, 11060 RSC.
- X. Qing, S. Liu, K. Huang, K. Lv, Y. Yang, Z. Lu, D. Fang and X. Liang, Electrochim. Acta, 2011, 56, 4985 CrossRef CAS PubMed.
- Z. Y. Lu, Z. Chang, J. F. Liu and X. M. Sun, Nano Res., 2011, 4, 658 CrossRef CAS PubMed.
- J. Huang, V. Meunier, B. G. Sumpter, P. M. Ajayan, J. J. Yoo, K. Balakrishnan, A. Srivastava, M. Conway, A. L. M. Reddy and J. Yu, Nano Lett., 2011, 11, 1423 CrossRef PubMed.
- J. Jiang, J. Liu, X. Huang, Y. Li, R. Ding, X. Ji, Y. Hu, Q. Chi and Z. Zhu, Cryst. Growth Des., 2009, 10, 70 Search PubMed.
- X. H. Lu, T. Zhai, X. H. Zhang, Y. Q. Shen, L. Y. Yuan, B. Hu, L. Gong, J. Chen, Y. H. Gao, J. Zhou, Y. X. Tong and Z. L. Wang, Adv. Mater., 2012, 24, 938 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |