Recent advancements in the production of hydroxymethylfurfural
Abstract
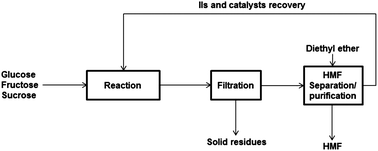
Lignocellulosic materials can be utilized in the production of platform chemicals such as hydroxymethylfurfural (HMF). HMF production has been investigated in various aqueous, solvent, biphasic and ionic liquid systems. Aqueous medium usually suffers from a low HMF yield (usually 50 to 60% while using fructose as feedstock) due to the production of by-products and the decomposition of HMF. A higher HMF production was achieved by applying biphasic systems, however, these systems face some technical challenges including solvent recovery, process complexity and environmental issues, which prevent its practical implementations at industrial scales. The unique properties of ionic liquids (IL)s make them promising solvents for producing HMF from polysaccharides. In this review, the effects of various parameters such as catalysts, solvents, and process conditions on the production of HMF from various lignocellulosic feedstocks as well as systems developed for purifying HMF after production are discussed. Generally, the yield of HMF production in the IL systems was higher than 80% when fructose was used as the raw material, but was less than 50% when cellulose or other polysaccharides were used. However, the IL system is complicated and has a challenging recovery process. The proposed IL systems are also not environmentally friendly. The main emphasis of this paper is on the industrial applicability of proposed processes for producing HMF.

 Please wait while we load your content...
Please wait while we load your content...