Protein–polymer co-induced exfoliated layered silicate structure based nanofibrous mats and their cytotoxicity
Xingyun Liu†
a,
Xiaoping Wang†b,
Jianwei Zhangc,
Xiankai Wangc,
Yuan Lua,
Hu Tua,
Hongbing Deng*c and
Linbin Jiang*a
aSchool of Chemistry and Chemical Engineering, Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology, Guangxi University, Nanning 530004, China. E-mail: jianglinbin@126.com; Fax: +86 771 3233718; Tel: +86 771 3239203
bDepartment of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University, Xi'an 710038, China
cSchool of Resource and Environmental Science, Wuhan University, Wuhan 430079, China. E-mail: hbdeng@whu.edu.cn; alphabeita@yahoo.com; Fax: +86 27 68778501; Tel: +86 27 68778501
First published on 3rd December 2013
Abstract
In this study, albumin egg (AE) induced layered rectorite (REC) to exfoliate by electrostatic forces between the molecular chains of AE and the interlayer of REC, which was confirmed by small angle X-ray diffraction. The composite nanofibrous mats with the exfoliated layered rectorite were successfully fabricated via electrospinning poly(vinyl alcohol) (PVA)/AE/REC solutions with different mass ratios. The morphology of the nanofibrous membranes was examined by field emission scanning electron microscopy. Energy-dispersive X-ray spectroscopy and Fourier transform infrared spectra showed the existence of REC in the composite nanofibrous mats. Besides, the wide angle X-ray diffraction and Fourier transform infrared spectra results demonstrated the crystalline structure of PVA was changed by formic acid. The differential scanning calorimetry and the thermo-gravimetric analysis results suggested that REC could slightly improve the thermal stability of the composite nanofibrous mats. MTT assay showed the nanofibrous mats were nontoxic and beneficial to the proliferation of L929 cells.
1. Introduction
In recent years, polymer–inorganic nanofibrous mats, which were fabricated by electrospinning technology, have drawn extensive attention. Not only did the composite nanofibrous mats possess ultrafine diameters and three dimensional (3D) nanofibrous structure, but they also had the optimal properties of both the polymer and the inorganic materials. Inorganic materials, such as silver nanoparticles,1 platinum (Pt) nanoparticles,2 quantum dots,3 carbon nanotubes4,5 and layered silicate etc.,6 were incorporated into the nanofibrous mats which were applied in bacterial inhibition,7,8 tissue engineering scaffolds,9 wound dressing,10 catalytic applications,11 cell culture12 and antitumor applications.13 Rectorite (REC) as a kind of layered silicate has a larger interlayer distance, better separable layer thickness and layer aspect ratio than regular montmorillonite (MMT).7 Besides, compared to pure polymers or conventional composites, the addition of REC could increase the modulus and strength, thermal stability, decrease gas permeability and flammability resistance.14–16 In addition, the European Food Safety Authority (EFSA) reported that bentonite (dioctahedral montmorillonite), another kind of layered silicate, was safe as a food additive.17 It was reported that nanofibrous mats with organic rectorite (OREC) had good cell compatibility.18 According to our previous reports,7,18,19 the polymer influences the layered structure of REC, as has been studied numerously, which showed that polymers could enlarge the distance between the interlayers of REC. However, our recent research about cellulose acetate (CA) nanofibrous mats coated with lysozyme-layered silicate composites by electrospraying,20 found that the REC was exfoliated by the positively charged lysozyme by electrostatic repulsion forces. Therefore, in order to further investigate the effect of protein on the layered structure of REC in electrospinning, albumin egg (AE) was selected as the protein additive in this study.AE, a single-chain phosphoglycoprotein, has excellent water solubility and is abundant in egg. It consists of 385 amino acid residues and its molecular weight is 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da.21 The AE molecule, with an ellipsoidal shape, possesses one internal disulfide bond and four free sulfhydryl groups.22 In addition, AE is low cost, non-toxic, biocompatible and biodegradable. Unfortunately, the formation of AE nanofibers fails during electrospinning, because the AE globular molecule has low polymeric solution entanglement.23
000 Da.21 The AE molecule, with an ellipsoidal shape, possesses one internal disulfide bond and four free sulfhydryl groups.22 In addition, AE is low cost, non-toxic, biocompatible and biodegradable. Unfortunately, the formation of AE nanofibers fails during electrospinning, because the AE globular molecule has low polymeric solution entanglement.23
Many natural and synthetic polymers, including gelatin, cellulose, silk fibroin, poly(ethylene oxide) (PEO), polystyrene (PS), poly(vinyl alcohol) (PVA) and poly (L-lactide) (PLLA), have been successfully applied for improving the electrospinability of other polymers. In order to successfully electrospin AE, PVA was selected as the polymer additive because of its good fiber forming, biocompatibility, and chemical resistance properties.24
In the process of electrospinning, the solvent vapour pressure and solvent volatility can greatly affect the evaporation rate, the drying time and the phase separation, which therefore makes them play significant roles in the formation of nanofibers.25 The evaporation rate of formic acid is 2.3 × 10−8 g mm−2 s−1 at room temperature (22 °C).26 Besides, it was reported that silk fibroin (SF) dissolved in formic acid and then the turbidity of the SF–formic acid solution was low and nearly constant even when the concentration of SF increased.27 In addition, for the unfolding and swelling of the globular whey protein β-lactoglobulin, the optimal conditions were with 98% formic acid as solvent.28 Moreover, in previous studies, galactosylated chitosan (GC) nanofibrous mats were electrospun with formic acid as solvent, and confirmed the hepatocytes cultured on the GC nanofibrous mats formed stable 3D spheroid aggregates and illustrated the cell bioactivity. In summary, formic acid was selected as the solvent in this work, as it could produce stable solutions and help to form excellent nanofibers.
In this study, PVA–AE and PVA–AE–REC composite nanofibrous mats were prepared via the electrospinning technique. Field emission scanning electron microscopy (FE-SEM), Fourier transform infrared spectroscopy (FT-IR) and energy-dispersive X-ray spectroscopy (EDX) were applied for analyzing the morphology and composition of the nanofibrous mats. Small angle X-ray diffraction (SAXRD) was performed to determine whether the layered structure of REC was present or exfoliated. The crystallization of the composite nanofibrous mats was analyzed by wide angle X-ray diffraction (WAXRD). Thermo-gravimetric analysis (TGA) and differential scanning calorimetry (DSC) investigated the thermal stability of the composite nanofibrous mats before and after the addition of REC. The cytotoxicity of the nanofibrous mats was measured by MTT assay.
2. Materials and methods
2.1. Materials
Poly(vinyl alcohol) (PVA, Mw = 9 × 104) was provided by Sigma Aldrich Chemical Reagent Co., USA. Calcium rectorite (Ca2+–REC) was purchased from Hubei Mingliu Co., China. Albumin egg (AE) was supplied by Sigma (USA) and formic acid (98%) was obtained from Sinopharm Chemical Reagent Co. (Shanghai China). Other chemicals of analytical grade were used as received without any further purification. All solutions were prepared using formic acid solutions.2.2. Preparation of electrospinning solutions and nanofibrous mats
8% PVA solution was prepared by dissolving PVA powder in 98% formic acid solution at room temperature with gentle magnetic stirring for 6 h. 5% AE solution was prepared by adding AE powder to 98% formic acid solution at room temperature. 8% PVA and 5% AE solutions were mixed in different mass ratios of PVA/AE at 80/20, 60/40, 50/50 and 40/60 under gentle magnetic stirring. 1% REC was added to each PVA–AE mixture solution, and then those blending solutions were stirred for 48 h at room temperature. The concentrations of all the above solutions are expressed in wt/wt%.The blending solutions of PVA–AE and PVA–AE–REC were fed into a glass syringe with a needle having an internal diameter of 0.8 mm. The glass syringe was fixed and driven by the syringe pump (LSP02-1B, Baoding Longer Precision Pump Co., Ltd., China) at a speed of 0.5 mL h−1. The applied voltage of 15 kV was supplied by a high voltage power supply (DW-P303-1ACD8, Tianjin Dongwen High Voltage Co., China), and the tip-to-collector distance was 10 cm. The as-spun fibers were collected by a grounded collector covered with aluminum foil. The ambient temperature and relative humidity were maintained at 25 °C and 45%, respectively. The prepared nanofibrous membranes were dried in vacuum at room temperature for 24 h to remove the trace solvent.
2.3. Characterization
The morphology of the nanofibrous membranes was examined by field emission scanning electron microscopy (FE-SEM, S-4800, FEI Ltd., Japan). All the nanofibrous mats were sputter coated with gold prior to FE-SEM imaging. The composition of the nanofibrous mats was investigated by energy-dispersive X-ray (EDX) spectroscopy (S-4800, FEI Ltd., Japan). Fourier transform infrared (FT-IR) spectra were recorded by Nicolet170-SX (Thermo Nicolet Ltd., USA). Small angle X-ray diffraction (SAXRD) and wide angle X-ray diffraction (WAXRD) were performed using diffractometer type D/max-Ra (Rigaku Co., Japan) with Cu target and Kα radiation (λ = 0.154 nm). The scanning rate and scope of 2θ of the SAXRD were 1° min−1 and 1–6°, while those of the WAXRD were 6° min−1 and 5–60°, respectively. The thermal property of the nanofibrous mats was determined by differential scanning calorimetry (DSC, 204 F1, Zetzsch, Germany) and thermo-gravimetric analysis (TGA, Pyris 1 TGA, PerKinElmer, USA). The DSC was heated up twice from room temperature to 500 °C and the heating rate was 10 °C min−1 under nitrogen flow (20 mL min−1) to remove the heat history. TGA was performed at a heating rate of 10 °C min−1 from room temperature to 500 °C under a flow of air.2.4. MTT assay
In line with a previous report,29 the cytotoxicity of the nanofibrous mats was measured by MTT (methylthiazolydiphenyl-tetrazolium bromide) assay, which generated a purple formazan product. The amount of formazan was proportional to the quantity of viable cells. In brief, L929 cells cultured on the nanofibrous mats on the culture plate were washed with phosphate buffered saline (PBS). After cultivation for 24 h and 72 h at 37 °C, 25 μL MTT was added to each plate and incubated for 4 h. Then, the MTT formazan purple crystals were dissolved by adding DMSO (150 μL) to each plate and waiting for 10 min. The absorbance of the solution was measured at 490 nm by an enzyme linked immunosorbent assay (ELISA) reader (MODEL550, Bio-Rad, USA). The results were presented as the mean ± standard deviation (SD). Significance testing was performed using one-way analysis of variance (ANOVA), followed by the LSD test. Values of *p < 0.05 and **p < 0.01 were considered significant.3. Results and discussion
3.1. Morphology of the fibrous mats
The morphology of electrospun nanofibers can be influenced by the electrospinning parameters, such as the concentration of the solutions, surface tension, electric voltage and tip-to-collector distance, etc.30 Fig. 1 shows the morphology of the PVA–AE nanofibrous mats with different mass ratios from 100/0 to 40/60. The PVA nanofibrous mats (Fig. 1a) displayed uniform nanofibrous shape and 3D structure, and the nanofibrous shape was extremely stable and continuous, attributable to the outstanding film-forming and spinnability properties of PVA. Interestingly, with decreasing content of PVA, the average diameter of the composite PVA–AE nanofibrous mats (Fig. 1b–e) was much smaller than that of the PVA nanofibrous mats (Fig. 1a). This is because the higher amount of PVA can lead to higher viscosity of the blending solution which favors the molecular entanglement necessary for nanofiber formation.10 The other possible reason was that solvent evaporation from the mixture solution droplet affected the formation of nanofibers.31 Besides, the nanofibers became straight and loose when AE was incorporated with PVA. In addition, some fractured nanofibers appeared among the PVA–AE composite nanofibrous mats. As we know, AE with lower strength and elasticity produces nanofibers that are brittle and break easily.32 | ||
| Fig. 1 FE-SEM images of nanofibrous mats electrospun from PVA–AE solutions at different mass ratios: (a) 100/0, (b) 80/20, (c) 60/40, (d) 50/50 and (e) 40/60. | ||
Fig. 2 presents the morphology of the PVA–AE–REC nanofibrous membranes at different mass ratios from 80/20 to 40/60 containing 1% REC. Correspondingly, the diameter and morphology of the PVA–AE–REC nanofibers (Fig. 2a–d) were similar to those of the PVA–AE nanofibrous mats (Fig. 1b–e) at the same mass ratios, respectively. When the content of AE was increased, some nanofibers were also fractured. Besides, the nanofibers with REC became disordered and entanglement among the nanofibers was remarkably observed. Comparing Fig. 1d with 2c, and Fig. 1e with 2d, the nanofibers became much thinner and the fractured nanofibers were enriched. The above results illustrated that REC with electrical charge resulted in the conductivity of the blending solution changing so the formation of the nanofibers was influenced, which is in agreement with our previous study.7
 | ||
| Fig. 2 FE-SEM images of nanofibrous mats electrospun from PVA–AE solutions at different mass ratios containing 1% REC: (a) 80/20, (b) 60/40, (c) 50/50 and (d) 40/60. | ||
3.2. Composition analysis of the nanofibrous mats
The existence of REC and AE in the PVA–AE–REC composite nanofibrous membranes was verified by EDX spectroscopy. Fig. 3 shows the EDX spectrum of the PVA–AE composite nanofibrous mats containing 1% REC. Actually, AE contains the element S, and the characteristic elements of REC are Si and Al. The characteristic peak of the element S was detected in the spectrum, which confirmed that the AE existed in the nanofibrous mats. In addition, the characteristic peaks of Si and Al were observed, which proved that REC had been successfully incorporated into the composite nanofibrous mats. | ||
| Fig. 3 EDX spectroscopy of a selected area from the nanofibrous mats electrospun from PVA–AE solutions with a mass ratio of 80/20 containing 1% REC. | ||
3.3. FT-IR spectra analysis
Fig. 4 shows the FT-IR spectra of the bulk materials, PVA nanofibrous mats and the electrospun composite nanofibrous mats. The REC exhibited characteristic peaks at 467 and 546 cm−1, which stood for the Si–O bending vibration band, 910 cm−1 due to the –OH vibration, 1025 and 1050 cm−1 assigned to the Si–O stretching vibration band, and 1650 cm−1 attributed to the H2O bending vibration.7 According to our previous reports,7,19 in the FT-IR spectra of PVA nanofibrous mats fabricated from electrospinning aqueous solutions, the absorption peaks at 1719, 1435, 1181 and 850 cm−1 belonged to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–H, C–O and C–C resonances, respectively. However, the peaks of C
O, C–H, C–O and C–C resonances, respectively. However, the peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1719 cm−1 and C–O at 1181 cm−1 became sharp and strong, possibly because the –COOH group of formic acid was bonded to the –OH of PVA and formed an ester. It implied that the structure of PVA was slightly changed. The characteristic peaks of AE at 1654 and 1540 cm−1, were assigned to the stretching bands of amide I and amide II. The peak at 1450 cm−1 resulted from the asymmetric bending vibration of –CH2.
O at 1719 cm−1 and C–O at 1181 cm−1 became sharp and strong, possibly because the –COOH group of formic acid was bonded to the –OH of PVA and formed an ester. It implied that the structure of PVA was slightly changed. The characteristic peaks of AE at 1654 and 1540 cm−1, were assigned to the stretching bands of amide I and amide II. The peak at 1450 cm−1 resulted from the asymmetric bending vibration of –CH2.
 | ||
| Fig. 4 FT-IR spectra of the bulk materials and nanofibrous mats with various PVA–AE mass ratios: (a) 100/0, (b) 80/20 and (c) 80/20 containing 1% REC. | ||
In the spectra of the PVA–AE (Fig. 4b) and PVA–AE–REC (Fig. 4c) nanofibrous mats, the peaks of amide I at 1650 cm−1 and amide II at 1540 cm−1 appeared, which confirmed that AE existed in the composite nanofibrous mats. Compared with the FT-IR spectrum of REC, the Si–O bending vibration at 546 and 467 cm−1 demonstrated that REC was successfully incorporated into the PVA–AE–REC nanofibrous mats (Fig. 4c). The result was identical with that of EDX, which further verified the existence of REC.
3.4. SAXRD and WAXRD patterns
In order to investigate whether the predesigned intercalated architecture existed in PVA–AE–REC nanofibrous mats or not, the SAXRD patterns of the bulk materials and nanofibrous mats were recorded (Fig. 5). In the SAXRD pattern of REC, the 2θ was 3.80°. According to the Bragg's equation, the interlayer distance was 2.32 nm. However, compared with the pattern of REC, the diffraction peak could not be observed in the curves of the PVA–AE–REC nanofibrous mats (Fig. 5b and c), which illustrated that REC was exfoliated.33 Based on our previous report, PVA chains could insert into the interlayer of layered silicate such as organic rectorite (OREC) and enlarge its distance but could not exfoliate it.7,18,19 | ||
| Fig. 5 SAXRD patterns of the bulk materials and nanofibrous mats with various PVA–AE mass ratios: (a) 100/0, (b) 80/20 containing 1% REC and (c) 60/40 containing 1% REC. | ||
The process of the exfoliation of REC is demonstrated in Scheme 1. The molecular chains of PVA could directly intercalate into the interlayer of REC.34 The isoelectric point (pI) of AE was 4.8.21 Because formic acid (98%) was selected as solvent in this work, the pH value of the blending solutions was below the value of the pI. As we know, the protein was positively charged when the value of the pH was below the pI value. In addition, REC was negatively charged.35 Consequently, the AE anchored to the REC galleries was attributed to the electrostatic interaction.36 Subsequently, the swelling of the molecular chains caused the distance of the interlayer to be larger. As a consequence, the layered structure was destroyed ultimately by the AE chains due to the electrostatic interactions between the positively charged AE and negatively charged REC.36
 | ||
| Scheme 1 Schematic diagram illustrating the fabrication of the nanofibrous mats and the exfoliation process of REC. | ||
Fig. 6 shows the WAXRD patterns of the bulk materials and nanofibrous mats. In the WAXRD pattern of REC, the crystalline peaks were 7.34°, 18.06°, 20.00°, 29.06° and 35.84°, respectively, and the crystalline peaks of AE were 8.72° and 21.98°. AE displayed broad peaks especially for the smaller diffraction angles, which suggested that long range disorder was found in the protein samples.37 Generally, the diffraction peaks of PVA consist of 12.5°, 20.00°, 23.2° and 40.9°, respectively.19 However, in Fig. 6, the single crystalline peak of the PVA nanofibrous mats (Fig. 6a) was at 26.92°, because the crystalline structure of PVA was destroyed when dissolved in the formic acid solution, which was consistent with the FT-IR result. Consequently, the PVA nanofibrous mats became mostly amorphous and the crystallinity became much lower.38 Besides, the crystalline peaks of the PVA–AE nanofibrous mats were 23.50° (Fig. 6b) and 21.38° (Fig. 6c), respectively. In the curves of Fig. 6a–c, the crystalline peaks were shifted toward smaller angles. It indicated that the addition of AE could affect the crystallization of PVA. The crystalline peak around 21.66° of the PVA–AE–REC composite nanofibrous mats became wider and stronger, which denoted that REC could influence the crystalline structure of PVA or AE. However, the peak of REC at 7.34° disappeared in the WAXRD pattern of the PVA–AE–REC nanofibrous mats because the molecular movement of PVA or AE was limited greatly due to the formation of the interaction structure.39
 | ||
| Fig. 6 WAXRD patterns of the bulk materials and nanofibrous mats with various PVA–AE mass ratios: (a) 100/0, (b) 80/20, (c) 60/40 and (d) 60/40 containing 1% REC. | ||
3.5. TGA and DSC assay
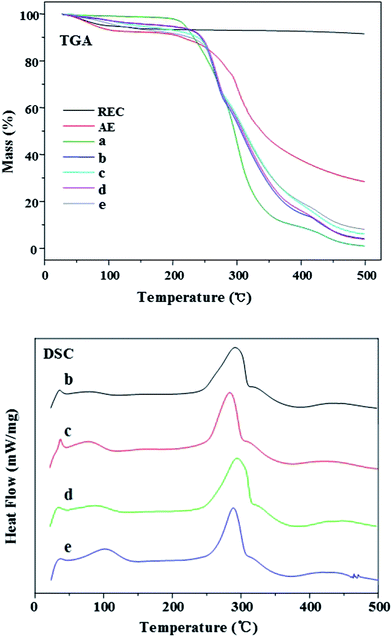
The thermal stability of the bulk materials and nanofibrous mats was measured by TGA. The curves of the TGA could display the moisture vaporization, the decomposition of biopolymers and the carbonization of the degraded products to ash.12 The conjugated polyene structure could be generated after the water was removed,30 and the three weight loss peaks of PVA nanofibrous mats (Fig. 7a) were as follows: the first one around 20–100 °C was due to water loss; the second one around 200–350 °C was attributed to the thermal decomposition of PVA; and the third one around 400–500 °C was due to the carbonization of the decomposed products to ash.19 Comparing the curves with one another, the mass loss of the nanofibrous mats increased with the increasing amount of PVA due to its poor thermal stability. It was clear that the degradation temperature of the composite nanofibrous mats (Fig. 7b–e) was between those of PVA and AE. Comparing Fig. 7b with d, as well as Fig. 7c with e, the mass loss of the nanofibrous mats without REC was more than those of the nanofibrous mats with REC, which revealed that the nanofibrous mats with REC exhibited better thermal stability, because the REC with high aspect ratio could strongly hinder the volatility of the degenerated polymer during pyrolysis.35In order to further evaluate the thermal properties, DSC was also recorded. The exothermic peaks of the composite nanofibrous mats with various PVA–AE mass ratios (Fig. 7b–e in the DSC spectra) were 291.9, 284.9, 294.9 and 287.9 °C, respectively. With the increasing amount of AE in the PVA–AE nanofibrous mats, the exothermic peaks tended to be small and the temperature of crystallization became relatively low. The reason was that the addition of AE affected the crystallization of PVA. Under the same weight ratios of PVA–AE, the addition of REC resulted in an increased temperature of crystallization. It revealed that REC with high temperature resistance could enhance the thermal stability of the nanofibrous mats.35 In the curves of the DSC, the melting temperature (Tm) of the nanofibrous mats was 386.9, 375.9, 382.9 and 371.9 °C, respectively. With increasing mass ratio of AE, the endothermic peaks of the composite nanofibrous mats became broad and shifted toward lower temperatures. The reason might be that a large number of molecular chains of PVA or AE were in the noncrystalline state during the rapid evaporation of solvent, which solidified the electrospun nanofibers during electrospinning.10 When the REC was incorporated into the nanofibrous mats, the melting temperature of the composite nanofibrous mats was not obviously changed, which demonstrated the addition of AE or REC had no remarkable effect on the melting temperature of the as-spun nanofibrous mats.
3.6. MTT assay
The cytotoxicity of the nanofibrous mats to L929 cells was evaluated by MTT assay. Fig. 8 exhibits the viability of L929 cells which were cultivated for 24 and 72 h. After cultivating L929 cells on the nanofibrous mats for 24 h, statistically significant differences in the cell activity could be observed in most of the MTT results. The viability of the cells among the nanofibrous mats (Fig. 8a–e) was improved in comparison with that of the control, which indicated that the nanofibrous mats were nontoxic and were good for the proliferation of cells.A similar phenomenon was observed after incubating the L929 cells for 72 h. However, the statistically significant difference in cell activity could be observed in the results of all the composite nanofibrous mats. Besides, for the PVA–AE nanofibrous mats (Fig. 8a–c), the cell viability was proportional to the content of AE. It illustrated that the cytotoxicity of the nanofibrous mats was gradually lowered with the addition of AE. This fact might be explained by the AE with good biocompatibility and it was available for the proliferation of cells. When REC incorporated into the nanofibrous mats, the cell viability was between that of the control and the nanofibrous mats without REC. It revealed that REC in nanofibrous mats had little cytotoxic effect on the L929 cells. This result is consistent with our previous studies.12,18
The MTT results indicated that all the nanofibrous mats were nontoxic to L929 cells and they could be beneficial for the proliferation of cells when the cells are incubated for 24 or 72 h on them, which is attributed to the excellent biocompatibility of PVA and AE. Consequently, these nanofibrous mats are good candidates for tissue engineering.
4. Conclusions
In this work, composite nanofibrous mats containing exfoliated REC were successfully fabricated via electrospinning a PVA/AE/REC mixture under formic acid solutions with different mass ratios. REC was exfoliated by electrostatic forces between the molecular chains of AE and the surface of REC, which was confirmed by small angle X-ray diffraction. The morphology of the nanofibrous mats was dramatically affected by the quantity of AE and REC. The results of EDX and FT-IR verified that the REC was successfully incorporated into the composite nanofibrous mats. The thermal stability of the composite nanofibrous mats was slightly improved by the addition of REC. In addition, formic acid, which was selected as solvent, resulted in the change of the crystalline structure of PVA. MTT assay results showed that the nanofibrous mats were nontoxic for L929 cells and were beneficial to the proliferation of cells, which implies the potential applications of these nanofibrous mats in tissue engineering.Acknowledgements
This project was funded by the National Natural Science Foundation of China (no. 31101365) and the Key Project of Natural Science Foundation of Guangxi Province of China (no. 2011GXNSFD018012), and partially supported by the National Basic Science Personnel Training Fund (no. J1103409). We are grateful to the staff from the Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University for the cell experiment assistance.References
- D. He, B. Hu, Q.-F. Yao, K. Wang and S.-H. Yu, ACS Nano, 2009, 3, 3993 CrossRef CAS PubMed.
- E. Formo, E. Lee, D. Campbell and Y. Xia, Nano Lett., 2008, 8, 668 CrossRef CAS PubMed.
- M. Li, J. Zhang, H. Zhang, Y. Liu, C. Wang, X. Xu, Y. Tang and B. Yang, Adv. Funct. Mater., 2007, 17, 3650 CrossRef.
- F. Ko, Y. Gogotsi, A. Ali, N. Naguib, H. Ye, G. Yang, C. Li and P. Willis, Adv. Mater., 2003, 15, 1161 CrossRef CAS.
- X. Wang, Y. Si, X. Wang, J. Yang, B. Ding, L. Chen, Z. Hu and J. Yu, Nanoscale, 2013, 5, 886 RSC.
- Y. H. Lee, J. H. Lee, I.-G. An, C. Kim, D. S. Lee, Y. K. Lee and J.-D. Nam, Biomaterials, 2005, 26, 3165 CrossRef CAS PubMed.
- H. Deng, X. Li, B. Ding, Y. Du, G. Li, J. Yang and X. Hu, Carbohydr. Polym., 2011, 83, 973 CrossRef CAS PubMed.
- D. Wang, W. Xu, G. Sun and B.-s. Chiou, ACS Appl. Mater. Interfaces, 2011, 3, 2838 CAS.
- S. Agarwal, J. H. Wendorff and A. Greiner, Adv. Mater., 2009, 21, 3343 CrossRef CAS PubMed.
- Y. Zhou, D. Yang, X. Chen, Q. Xu, F. Lu and J. Nie, Biomacromolecules, 2007, 9, 349 CrossRef PubMed.
- X. Fang, H. Ma, S. Xiao, M. Shen, R. Guo, X. Cao and X. Shi, J. Mater. Chem., 2011, 21, 4493 RSC.
- R. Huang, Y. Li, X. Zhou, Q. Zhang, H. Jin, J. Zhao, S. Pan and H. Deng, Carbohydr. Polym., 2012, 90, 957 CrossRef CAS PubMed.
- M. G. Ignatova, N. E. Manolova, R. A. Toshkova, I. B. Rashkov, E. G. Gardeva, L. S. Yossifova and M. T. Alexandrov, Biomacromolecules, 2010, 11, 1633 CrossRef CAS PubMed.
- E. P. Giannelis, Appl. Organomet. Chem., 1998, 12, 675 CrossRef CAS.
- J. W. Gilman, Appl. Clay Sci., 1999, 15, 31 CrossRef CAS.
- S. Sinha Ray and M. Bousmina, Prog. Mater. Sci., 2005, 50, 962 CrossRef PubMed.
- European Food Safety Authority, EFSA J., 2011, 9, 2007 Search PubMed.
- S. Xin, Y. Li, W. Li, J. Du, R. Huang, Y. Du and H. Deng, Carbohydr. Polym., 2012, 90, 1069 CrossRef CAS PubMed.
- W. Li, X. Li, Y. Chen, X. Li, H. Deng, T. Wang, R. Huang and G. Fan, Carbohydr. Polym., 2012, 2232 Search PubMed.
- W. Li, X. Li, Q. Wang, Y. Pan, T. Wang, H. Wang, R. Song and H. Deng, Carbohydr. Polym., 2014, 99, 218 CrossRef CAS PubMed.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 157, 168 CrossRef CAS PubMed.
- Y. Mine, Trends Food Sci. Technol., 1995, 6, 225 CrossRef CAS.
- S. Wongsasulak, M. Patapeejumruswong, J. Weiss, P. Supaphol and T. Yoovidhya, J. Food Eng., 2010, 98, 370 CrossRef CAS PubMed.
- Y. Zhou, D. Yang, X. Chen, Q. Xu, F. Lu and J. Nie, Biomacromolecules, 2008, 9, 349 CrossRef CAS PubMed.
- N. Bhardwaj and S. C. Kundu, Biotechnol. Adv., 2010, 28, 325 CrossRef CAS PubMed.
- K. McGrath and D. Kaplan, Protein-based materials, Springer, 1997 Search PubMed.
- I. C. Um, H. Y. Kweon, K. G. Lee and Y. H. Park, Int. J. Biol. Macromol., 2003, 33, 203 CrossRef CAS PubMed.
- M. van der Leeden, A. Rutten and G. Frens, J. Biotechnol., 2000, 79, 211 CrossRef CAS.
- X. Yang, X. Chen and H. Wang, Biomacromolecules, 2009, 10, 2772 CrossRef CAS PubMed.
- M. S. Islam and M. R. Karim, Colloids Surf., A, 2010, 366, 135 CrossRef CAS PubMed.
- A. S. Nain, J. C. Wong, C. Amon and M. Sitti, Appl. Phys. Lett., 2006, 89, 183105 CrossRef PubMed.
- S. Zhong, W. E. Teo, X. Zhu, R. W. Beuerman, S. Ramakrishna and L. Y. L. Yung, J. Biomed. Mater. Res., Part A, 2006, 79A, 456 CrossRef CAS PubMed.
- S. Pavlidou and C. D. Papaspyrides, Prog. Polym. Sci., 2008, 33, 1119 CrossRef CAS PubMed.
- F. Bergaya, M. Jaber and J.-F. Lambert, in Environmental Silicate Nano-Biocomposites, Springer, 2012, p. 41 Search PubMed.
- X. Wang, B. Liu, J. Ren, C. Liu, X. Wang, J. Wu and R. Sun, Compos. Sci. Technol., 2010, 70, 1161 CrossRef CAS PubMed.
- A. J. Poole, J. S. Church and M. G. Huson, Biomacromolecules, 2008, 10, 1 CrossRef PubMed.
- S. Tripathi, G. Mehrotra and P. Dutta, Carbohydr. Polym., 2010, 79, 711 CrossRef CAS PubMed.
- C. S. Ki, D. H. Baek, K. D. Gang, K. H. Lee, I. C. Um and Y. H. Park, Polymer, 2005, 46, 5094 CrossRef CAS PubMed.
- X. Wang, Y. Du, J. Luo, J. Yang, W. Wang and J. F. Kennedy, Carbohydr. Polym., 2009, 77, 449 CrossRef CAS PubMed.
Footnote |
| † Co-first author with the same contribution to this work. |
| This journal is © The Royal Society of Chemistry 2014 |