Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence†
Abstract
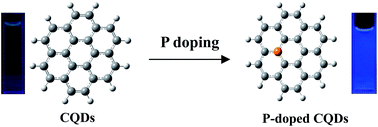
P-doped carbon quantum dots (PCQDs) were synthesized by a solvent-thermal method using phosphorous tribromide and hydroquinone as precursors. The as-prepared PCQDs present strong visible fluorescence with quantum yield up to 25%. The toxicity and bioimaging experiments showed that PCQDs have low cell toxicity and excellent biolabeling ability.

 Please wait while we load your content...
Please wait while we load your content...